Safety and Tolerability of NegEnt, a New Botanical Product Based on Liposomal Cannabidiol: A Case Series Report
Tullio Scrimali
Department of Medical, University of Catania and ALETEIA Lab for Medical Cannabis, Catania, Italy
Published Date: 2024-08-29DOI10.36648/ipctn.9.4.35
Tullio Scrimali*
Department of Medical, University of Catania and ALETEIA Lab for Medical Cannabis, Catania, Italy
- *Corresponding Author:
- Tullio Scrimali
Department of Medical,
Chirurgical Sciences and Advanced Technologies,
University of Catania and ALETEIA Lab for Medical Cannabis, Catania,
Italy,
Tel: +39095492945;
E-mail: tscrimact@gmail.com
Received date: June 09, 2023, Manuscript No. IPCTN-23-16954; Editor assigned date: June 12, 2023, PreQC No. IPCTN-23-16954 (PQ); Reviewed date: June 19, 2023, QC No. IPCTN-23-16954; Revised date: June 22, 2023, Manuscript No. IPCTN-23-16954 (R); Published date: August 29, 2024, DOI: 10.36648/ipctn.9.4.35
Citation: Scrimali T (2024) Safety and Tolerability of NegEnt, a New Botanical Product Based on Liposomal Cannabidiol: A Case Series Report. J Nutraceuticals Food Sci Vol.9.No.04: 35.
Abstract
The article describes a scientific study, carried out according to the case series report design involving 140 subjects, consisting of people who consumed NegEnt, an original, liposomal cannabidiol-based preparation in drops for aromatherapy, for at least one month and up to one year. The dose, taken daily, was 60 milligrams (six drops, in two pro-die administrations, of three drops each, sublingually). The study's objective was to assess the safety and tolerability of NegEnt in the medium and long term (one month and one year).
The research carried out also included a third arm, implemented according to the design of the single case research study. A volunteer, the author of this article (male, age 69), took 120 drops of NegEnt, diluted in 250 ml lowmineral water, in the early morning hours before breakfast.
The results, derived from the three arms of the research, described in the article, demonstrate the high safety and tolerability of NegEnt, confirming the literature data on cannabidiol, which demonstrate its very positive toxicological profile.
Only one adverse event, during the course of the research, was observed, at the start of treatment, in a single subject, who had previously suffered from multiple allergies and intolerances. This was a mild skin erythema, which resolved completely immediately after it was reported to the patient's doctor, who recommended discontinuing NegEnt, thereby resolving the problem.
Keywords
NegEnt; Liposomal cannabidiol; CBD-based nutraceuticals; Safety; Tolerability
Introduction
NegEnt is a scientifically validated, cannabidiol-based herbal aromatherapy preparation capable of improving human health, made water-soluble by conjugation with edible liposomes, thanks to the implementation of a high-pressure emulsification process. NegEnt was developed and filed by Scrimali, at the patent office of the city of Milan [1-3]. It is a plant derivative, obtained by the supercritical carbon dioxide method, from Cannabis sativa L., an industrial variety, legally cultivated in Italy. NegEnt does not contain THC or other cannabinoids. It is therefore a preparation based on isolated cannabidiol.
Currently, the regulatory framework Herbal Neurocare refers to, for the marketing of NegEnt liposomal, in Italy, is that of herbal product for aromatherapy, available over the counter, obtained, by distillation, from the essential oil of Cannabis sativa.
The guarantees of efficacy and absence of risks were traceable, at the time of NegEnt's release on the market, via an electronic shop, set up on Herbal Neurocare's corporate website (www.herbalneurocare.it), which took place in April 2022, to over fifteen years of official use of cannabidiol, as a plantderived drug, in a European country, the Netherlands, and over thirty years of use in Jamaica, as well as on the basis of the comprehensive monograph, published in 2018, by the World Health Organization [4]. It was also important that Epidiolex, a cannabidiol-based drug, had been approved, both in the United States, by the Food and Drug Administration (FDA) and in the European Union, by the European Medicines Agency (EMA), for the treatment of certain forms of epilepsy, having demonstrated high therapeutic activity and tolerability (Epidiolex). Several recent scientific publications had also confirmed the tolerability of cannabidiol [5].
The purpose of this article is to illustrate a research study, carried out by the author, to evaluate, in scientifically valid terms, the tolerability and safety of the specific NegEnt product, with the adoption of a robust experimental design, traceable to the case series research study. The research carried out was divided into three arms, as follows: Evaluation of medium- and long-term tolerability and safety (one month and one year; arms no.1 and no. 2) and evaluation of the possible negative effects of an overdose (arm no. 3). The research activity on NegEnt was communicated to the Italian Ministry of Health and authorized through the silence-consent process.
Case Presentation
Arm no: 1
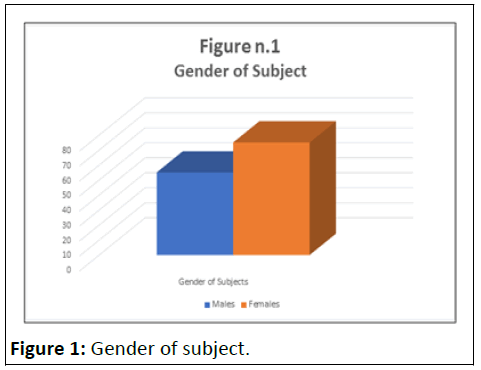
This included 130 patients, (55 males and 75 females; mean age 43 years with an age range of 14–71 years) who took NegEnt at the dosage of 60 mg per day for 30 days. These were subjects who spontaneously purchased NegEnt on the website www.herbalneurocare.it. Purchasers of NegEnt, who registered on the Herbal Neurocare website, in full compliance with Italian privacy legislation, were sent an email containing a single question regarding their intention to report any side effects or, in any case, functional and/or clinical problems that appeared during the period of taking NegEnt. In the email, the subject was asked to send a response to herbalneurocare@gmail.com choosing between two options: Absence of side effects or presence of side effects. In this case, they were asked to specify which side effects. The first 150 registered subjects were contacted. 130 people responded (as already mentioned, 55 males and 75 females; average age 43 years with an age range of 14–71 years).
Arm no: 2
The study included 10 patients (4 males and 6 females; average age: 46 years; range: 17–75 years) who, after purchasing NegEnt, were monitored by sending the same email every month for one year, for twelve months, identical to that sent, once only, to the subjects in arm no. 1 of the study. These subjects were asked to give informed consent to participate in the study, which would entail receiving one email per month, with the stipulation that they could withdraw from the research at any time and no longer receive emails monitoring the tolerability and safety profile of NegEnt.
Arm no: 3
The author of this article (male, age 69), took 120 drops of NegEnt, diluted in 250 ml low-mineral water, in the early morning hours before breakfast. Systolic and diastolic blood pressure, heart rate, peripheral blood oxygen saturation, appetite, digestion and sleep were monitored over 24 hours after NegEnt intake. The aforementioned biological parameters were recorded every hour, for the first 16 hours; recordings were suspended overnight and concluded at the end of 24 hours after NegEnt ingestion.
Transaminases were assessed the day before the NegEnt overdose experiment, after three days and after one month.
Results
Arm 1 of the study
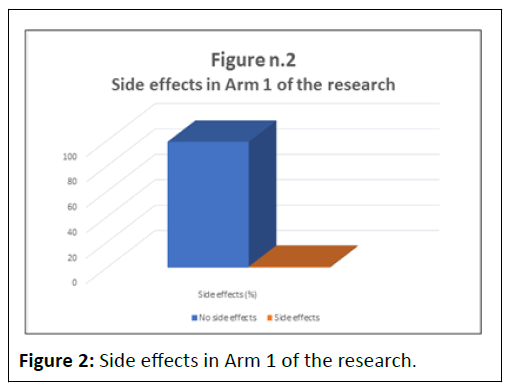
Of the 130 subjects who participated, only one reported an adverse event, consisting of the appearance of a skin erythema on the chest after the first day of therapy. Discontinuation of NegEnt caused the phenomenon to promptly recede.
The absence of side effects, for NegEnt, based on the application of the sign test was significant at p<0.01% (Tables 1 and 2) (Figures 1 and 2).
| Males | Females |
|---|---|
| 55 | 75 |
Table 1: Gender of subjects.
| No side effect | Side effects |
|---|---|
| 99,2 | 0,8 |
Table 2: Side effects in Arm 1 of the research.
Arm 2 of the study
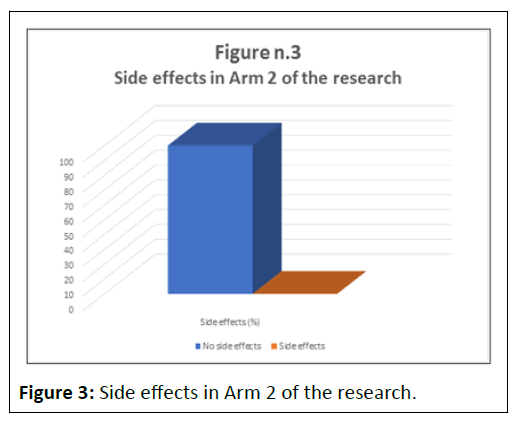
No side effects were reported in the 10 subjects who were monitored for one year (p<0.001%) (Table 3 and Figure 3).
| No side effect | Side effects |
|---|---|
| 100 | 0 |
Table 3: Side effects in Arm 2 of the research.
Arm 3 of the study
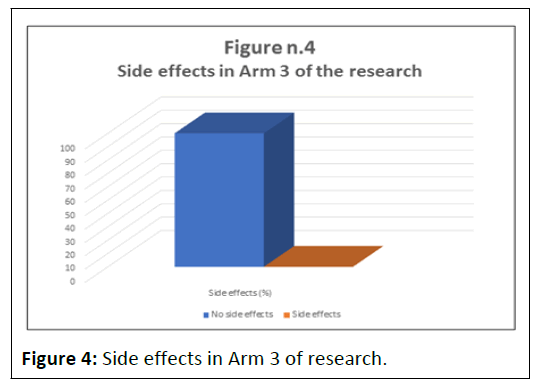
No significant changes in the monitored parameters were observed after the acute intake of 1200 mg of NegEnt, assessed by the Simulation Modelling Analysis Test.
Arterial blood pressure, systolic and diastolic blood pressure, heart rate, peripheral blood oxygen saturation, appetite, digestion and sleep at night did not show any significant changes, demonstrating NegEnt's tolerability even in the event of an overdose (p<0.05%) (Table 4 and Figure 4).
| No side effect | Side effects |
|---|---|
| 100 | 0 |
Table 4: Side effects in Arm 3 of the research.
Discussion
Research conducted on NegEnt to assess the tolerability and safety of this new herbal aromatherapy product, based on liposomal cannabidiol, derived from Cannabis Sativa L. and taken sublingually or ingested, demonstrated a very safe and reliable profile. In fact, there were no side effects in the medium term (one month of intake) or in the long term (one year). Even acute ingestion of a dose of 1200 mg (120 times the expected daily dosage) did not show any negative effects on the most important biological parameters.
NegEnt therefore proves to be a safe and manageable aromatherapy product based on liposomal cannabidiol with no side effects.
The only case of allergy that appeared suggested the advisability of including a warning in the package leaflet that NegEnt was associated with the appearance of reversible skin erythema in rare cases.
Conclusion
In this circumstance, it is suggested that you immediately stop taking the product, notify your family doctor and report the event to Herbal Neurocare, using the telephone numbers and email addresses provided on the company website and in the package leaflet.
Conflict of Interest
The author of the article is the Scientific Director and Founder of Herbal Neurocare SRL, Manufacturer of NegEnt.
References
- Scrimali T (2020) NegEnt: A cannabidiol-based, herbal medicine. Theoretical aspects, pharmacology, clinical and research perspectives, economic and social implications. Int J Herb Med 8: 143-151.
- Scrimali T (2022) NegEnt: Liposomal cannabidiol in human and veterinary medicine. Enna: ALETEIA Publisher Editions.
- Scrimali T (2023) Cannabidiol, NegEnt and panic disorder. Preprints.org.
- World Health Organisation (2018) Cannabidiol (CBD) Critical Review Report. Expert Committee on Drug Dependence, Fortieth Meeting, Geneva: 4-7.
- Pauli CS, Conroy M, Vanden Heuvel BD, Park SH (2020) Cannabidiol drugs clinical trial outcomes and adverse effects. Front Pharmacol 11: 63.
[Crossref],[Google Scholar],[Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences