Isolation of Essential Oil from Brassicca oleracea (Kale Leaves) and Characterization of Extract
1Department of Food Science and Technology, Jinnah University for Women, Karachi, Pakistan
2Department of Food Science and Technology, English Biscuits Manufacturer, Korangi Industrial Area, Karachi, Pakistan
3Department of Food Science and Technology, University of Karachi, Pakistan
- *Corresponding Author:
- Syeda Hafsa Aamir
Department of Food Science and Technology
Jinnah University for Women, Karachi, Pakistan
Email: s.seemajuw@gmail.com
Received Date: July 24, 2019; Accepted Date: August 27, 2019; Published Date: September 10, 2019
Citation: Aamir SH, Butt AY, Ali R, Ashraf S (2019) Isolation of Essential Oil From Brassicca oleracea (Kale Leaves) and Characterization of Extract. J Nutraceuticals Food Sci Vol. 4 No.1:4.
Copyright: © 2019 Aamir SH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Food Components having disease targeting and healing effects play a major role in diet therapy. Bioactive such as Dietary fibers, antioxidants and microbial are mostly provided from fruits and vegetables that are beneficial and various epidemic research has proven that daily intake of Brassica vegetables is highly correlated in minimizing cancers risk. The objective of this research was to isolate essential oils obtained from Kale leaves, characterization of the extract for Radical scavenging activity. From DPPH antioxidant activity the results indicated inhibition 80% of oxidative damage. The highest activity was obtained in Acetone washing. Essential oils extractions play diverse role in market for variety of processed foods. These essential oil properties allow its uses to consume foods and medicinal supplements. The extensive production and commercialization of kale leaves can be a realistic approach to increase the daily intake of antioxidants for prevention and easy to use application that may promote cure of variety of diseases.
Keywords
Kale leaves; Essential oil; Phytochemical screening; Crystallization; Antioxidant activity
Introduction
Plants play an important role in the development of potent therapeutic agents. The consumption of leafy vegetables is increasing day by day as a part of a healthy lifestyle. It contains a multitude of different molecules that act synergistically on targeted elements of the complex cellular pathway, thus, have been a source of a wide variety of biologically active compounds. They have been used extensively for crude material or as pure compounds for treating various diseases. Plants possess variety of free sugars, organic acids, amino acids, essential oils, lipids and minerals and they play an important role in human diet. Kale is thus can be considered as a functional food. Kale is a nutrient-dense, dark green leafy vegetable. It was one of the most common green vegetables in Europe. Current observations claimed that a diet containing fruits and vegetables is corresponding with a detain of the aging process and a subside risk of commencing oxidative stress and inflammation regarded to chronic diseases, such as neurological diseases, cataracts, cardiovascular diseases, cognitive function disorders, diabetes and atherosclerosis including Alzheimer’s [1].
Besides the Brassica oleracea L varieties, kale was competently examined and considered as essential bioactive compounds, in addition to glucosinates and phenolics. Phytochemical analysis was intended as the most important resource for information on analytical and high technology procedure in the food research sectors, plant sciences, etc. Moreover, phenolic compounds are claimed to possess antioxidant, anti-analgesic, anti-inflammatory properties and can, therefore, prevent diseases at risk. The natural antioxidants, more recently, have attracted considerable attention of users and researchers largely on account of adverse toxicological reports on some synthetic antioxidants and growing awareness among consumers.
DPPH Activity has been extensively studied regarding green vegetables and also improved as Nutraceuticals aspects of many health benefits.
The vegetables that contain the largest concentration of health-promoting sulfur-containing phytonutrients that increases the liver’s ability to produce enzymes that neutralize potentially toxic substances. Kale is also rich in the powerful phytonutrient antioxidants lutein and zeaxanthin. Benefits stated are related by the presence of Bioactives like phenolics and carotenoids. Additionally, the existence of Vitamin C, E, and fiber in fruits and vegetables also remarkably bestowed to these health benefits [1-5].
In fact, a single plant may have a diversity of phytochemical ranging from bitter compounds that stimulate digestive system, phenolic compounds for antioxidant and many other pharmacological properties, including antibacterial and antifungal, tannins that work as natural antibiotics, diuretic substances, and alkaloids. Essential oils, on the other hand, are ambrosial and inconstant volatiles excerpt from plants. These volatile compounds belong to various chemical classes: alcohols, ethers or oxides, aldehydes, ketones, esters, amines, amides, phenols, heterocycles, and mainly the terpenes. Alcohols, aldehydes, and ketones offer a wide variety of aromatic notes, such as fruity (E)-nerolidol), floral (Linalool), citrus (Limonene), herbal (γ-selinene), etc. The chemicals in essential oils are secondary metabolites, which plays a consequential function in plant defense as they retain antimicrobial characteristics.
Fortification and enrichment of essential oils are significantly being done in foods and drugs to improve dietary patterns and eliminate the prevailing disease. Herbal medicines derived from plant extracts are being increasingly utilized to treat a wide variety of clinic.
Materials and Methods
The leaves of Brassica Oleracea (kale) were used as a starting material was purchased from the grocery store at Karachi. 1, 1 Diphenyl-2-picryl-hydrazyl (DPPH) was purchased from Sigma-Aldrich (USA). Stock solutions of DPPH were prepared in methanol respectively. All the chemicals used in the experiment were of the analytical grade.
Isolation of essential oil
One Kg sample of fresh kale leaves were taken. The essential oil from fresh kale leaves was extracted by the technique of steam distillation using clevengers' Apparatus. Briefly describing 750 gm of the dried leaves were filled in 200 ml Clevenger’s Flask and 1 L water was taken in R.B Flask and then placed on a heating mantle of 1L. Chilling temperature provided to the chiller attached to apparatus was between 8-10°C for 6 hours. The distillate was collected with oil content in separate conical flask. The volume of hydrosol obtained was then measured. Using separating funnel of 500 ml, layers of oil from hydrosol was separated. 10 ml Hexane was added to the separating funnel along containing hydrosol and oil for better yield of essential oil. Flask was then shaken vigorously. The sample was left for 1 day. 20 ml pet ether and 20 ml distilled water was transferred to the separating funnel, and then the funnel was kept at rest for 10 minutes. The layers were collected from distillate and exercise was repeated three times for better yield. The solvent layers were discarded. 10 ml measuring cylinder was used to measure oil content. The obtained essential oil was preserved in an amber bottle.
Characterization and crystallization of extract
Crystallization and extraction of oil by the different solvent. One kg sample of kale Leaves was taken. The sample was soaked in 1000 ml methanol in 1000 ml beaker. The sample was covered using paraffin/aluminum foil and kept in dark. The sample then placed in fuming hood for evaporation of solvent for about 1 week. After 1 week, gummy solution was obtained for further evaporation of solvents; sample was allowed to run on a rotary evaporator. The desired Boiling point for evaporation of solvent (methanol) was 60ÃÆáÃâõÃâââ¬â¢C for 1hr whereas chilling temperature provided to the chiller attached to rota vapor was 4-10ÃÆáÃâõÃâââ¬â¢C. Speed of pump attached to chiller was at 3000-4000 rpm. Evaporation of solvent takes place. The concentrate was transferred to conical flask. The volume of concentrate obtained was measured and sample was preserved in dark. The methanolic extract obtained from Kale leaves was investigated in solvents that are polar and nonpolar in nature to identify components on UV VIZ Spectrophotometer.
The concentrated methanolic extract was transferred to a separating funnel of 250 ml. Washings were done using solvents such that ethyl acetate10%, Acetone 10%, ethanol 10% was added to separating funnel containing methanolic extract.
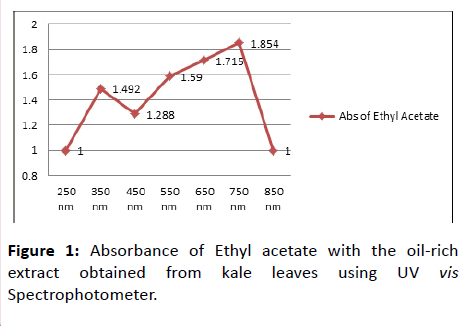
Then whole content was shake continuously for 20 minutes and then left for a few more minutes for settling in order to separate layers. Washing was done for multiple numbers of times to obtained a desired layer of solvent from concentrate. First washing layer of Ethyl acetate was separated from concentrate. Second washing acetone layer was obtained from concentrate. Third washing ethanol layer was separated from concentrate. All the three obtained layers obtaining oil content from methanolic extract were weight and spread on Petri dish to dry. Drying results in evaporation of solvents leaving sticky product showing oily appearance as well as aroma [6]. The oil by-product was allowed to cool. Oil was crystallized by the method given in their patent. No absolute crystals were formed on scratching the content in Petri dish. Briefly, acetone, n-hexane, ethyl acetate, ethanol, were added to the product again with 4:20 ratio as shown in Table 1. The oil content was then determined on a spectrophotometer, where 4 g of sticky less crystallize oil product was taken and added 20 ml of respective solvent to it. Absorbance was taken at a range of 250-850 nm as shown in Figure 1.
| S. No | Sequential Extraction | Color of the extract | Consistency |
|---|---|---|---|
| 1 | Ethanol (50%) | Yellowish Brown | Clear Liquid |
| 2 | Aqueous (after evaporation of Ethanol) | Dark brownish yellow | Turbid |
| 3 | Chloroform (100%) | Yellowish-brown | Clear liquid |
| 4 | Ethyl acetate (100%) | Pale yellow | Clear liquid |
| 5 | n-Butanol (100%) | Pale yellow | Clear liquid |
| 6 | Aqueous (after evaporation of organic solvents) | Light yellowish-brown | Clear liquid |
Table 1: Physical appearance of the sequential extraction.
Quantization of leaves extract containing oil
The absorbance of ethyl acetate with oil-rich extract obtained from kale leaves via U.V VIS Spectrophotometer proves to be increasing with increasing concentration. Chlorophyll (Chl a, Chl b) absorbs in the narrow bands (maxima) in the blue near 428 and 453 nm range and red near 661 and 642 nm spectral ranges [7].
Phytochemical screening
Phytochemical screening of all the extracts has been studied by various qualitative screening methods.
Total Flavonoids positive determination was carried out by Alkaline Reagent Test, Lead Acetate Test and Ferric Chloride Test. Terpenoids were investigated by Salkowski’s Test and were found to be positive. Successful determination for Phenolics by lead acetate test and ferric chloride test was done by using protocols and Glycoside content was estimated by Keller killni Test [8,9].
Mayer’s Reagent, Hager’s Test accesses for determination of Alkaloids.
Flavonoids:
• Alkaline reagent test: Incorporate a few drops of hydroxide solution to the test tube containing extract. Formation of intense yellow color will take place. Dilute with acid the sample in the test tube now becomes colorless Indicated the presence of flavonoids. The result of this test was found to be positive
• Lead acetate test: Add a few drops of lead acetate solution in a test tube containing extract. Formation of yellow precipitates takes place. Presence of flavonoids was confirmed. Result obtained was positive
• Ferric chloride test: Add a few drops of Ferric Chloride solution to the extract containing test tubes. Formation of intense green color confirmed the presence of flavonoids [8-10]
Test for terpenoids:
• Salkowski’s test: We added 2 ml H2SO4 concentrated to the whole aqueous extract Test tube sample turns color to reddish-brown. It showed the presence of phytosterols in the sample. A positive result was obtained
Determination for phenolics:
• Lead acetate test: Treat the extract with few drops of lead acetate solution. Formation of yellow precipitates indicated the presence of flavonoids thus result was positive
• Ferric chloride test: Incorporate few drops of Ferric Chloride solution to the test tube containing a sample. Development of intense green color takes place within a test tube sample. The color change indicated the presence of flavonoids [11-15]
Detection of glycosides:
• Keller killni test: Take few ml of extract as a sample in a test tube. Add 1ml concentrated sulphuric Acid. Appearance of the brown ring at the interface indicated the presence of glycosides. The appearance of a violet ring below the brown ring and a greenish ring in test tube confirmed the test
Reagents: Mayer’s Reagent, Hager’s Test.
• Mayer’s test: Filtrate is treated with potassium mercuric iodide solution (Mayer’s reagent). Formation of whitishyellow or cream-colored precipitates indicated the presence of alkaloids
• Hager’s test: Filtrate was treated with a saturated aqueous solution of picric acid Formation of yellow color precipitates alkaloids were confirmed (Table 2) [16]
| Sr. No. | Constituents | Tests | Results |
|---|---|---|---|
| 1 | Flavanoids | Lead acetate test | Results obtained from the test were found to be positive. |
| Alkaline reagent test | |||
| Ferric chloride test | |||
| 2 | Phenolics | Lead acetate test | |
| Ferric chloride test | |||
| 3 | Terpenoids | Salkowski test | |
| 4 | Alkaloids | Mayer’stest | |
| Hager’s test | |||
| 5 | Glycosides | Keller killni test |
Table 2: Phytochemical screening of extract obtained from Brassicae Oleracea (kale leaves) Standard Procedures by: Sofowara (1993), Trease and Evans (1989), Harborne (1973).
DPPH antioxidant assay
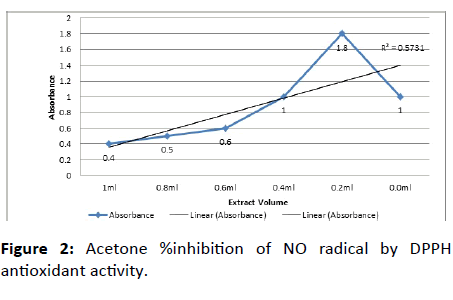
The nitric oxide scavenging activity was assayed as described earlier Alam et al., with some modifications. The sample solution was prepared by 1 gm/ml of oily extract in 10 ml methanol followed by centrifugation for 10 minutes at high speed, with different concentration ranging from 0.2-1 ml or 20-100 mg/ml poured into multiple test tubes. In each test tube, 1 ml of DPPH solution was added and make up the test tubes with methanol up to 2 ml and incubated at room temperature in dark for 15 minutes. After 15 minutes absorbance was taken at 517 nm as shown in Figure 2 and Table 3.
| Sr. No. | Amount | Abs |
|---|---|---|
| 1 | 1 ml | 0.4 |
| 2 | 0.8 ml | 0.5 |
| 3 | 0.6 ml | 0.6 |
| 4 | 0.4 ml | 1 |
| 5 | 0.2 ml | 1.8 |
| 6 | 0.0 ml | 1 |
Table 3: Acetone %inhibition of NO radical by DPPH antioxidant activity.
Absorbance was measured at 517 nm. Methanol was taken as positive control and it was calculated by:
A control-A test/A control × 100
Where A control represents the reading of control reaction and A test absorbance of the extract rich oil.
• Stock solution: 0.04 gm DPPH compound in 100 ml methanol
• Reference sample: Methanol• The antioxidant activity was measured by a plot between absorbance and sample concentration
• Nitric oxide radical inhibition: The nitric oxide radical inhibition was calculated by the equation
%Inhibition of NO radical= [Ao–A1]/Ao × 100
Results and Discussion
Isolation of essential oil
Essential oil composition is affected by various factors including temperature, irradiance, relative humidity, cultivation and photoperiod practices. The impact of extraction method on composition of oil and the accountability of the components of essential oil describe how the composition of the product achieved by steam distillation is frequently unlike from that which is originally present in the secretary organs of the vegetable. Distillate contained essential oil content up to 5 ml. Pet ether can be used to isolate the remaining amount of essential oil from distillate. The obtained essential oil was preserved in amber bottle.
Crystallization of essential oil components
After drying, the oily product was exposed to crystallization for the process of purification. It then undergoes crystallization at room temperature but not completely structured. It was partially crystallized. Crystals can be used in food preparation in the food industry. With the increasing trend for preference of natural products to cure and utilization as a food component, Essential oils are known to exhibit positive effects from commercial and economic aspects.
Quantization of leave extract using oil components
In Figure 1 Absorbance of ethyl acetate with oil-rich extract obtained from kale leaves via U.V VIS Spectrophotometer showed to be increasing with increasing concentration. The quantitative determination of chlorophyll (Chl a, Chl b), and carotenoids in a whole-pigment extract of green plant tissues by U.V-VIS spectroscopy is complicated by the choice of sample, solvent system and spectrophotometer used. Chlorophyll (Chl a, Chl b) absorbs in the narrow bands (maxima) in the blue near 428 and 453 nm range and red near 661 and 642 nm spectral ranges. Other compounds can also be studied if known ranges are provided.
Phytochemical screening
Phytochemical screening of components was performed over kale leaves. Medicinally active components obtained from the Brassica oleracea (kale) leaves extract indicated positive presence of flavonoids, alkaloids, phenolic compounds, tannins, terpenoids, glucosinates as showed in Table 2. They proved to exhibit antioxidants properties. Hence, it is capable of maintaining diet and can assist in preventing disease functioning as hepatotoxicity, neurodegenerative disorders, and chronic diseases.
DPPH antioxidant activity of methanolic extracts of Brassica oleracea (Kale leaves)
The DPPH scavenging activity was found to be concentration-independent. An inverse relation was observed. At concentration 0.2 ml, the %scavenging activity was 80% and as the concentration rose by 1 ml the scavenging capacity observed was 60% as shown in Figure 2. The % inhibition of DPPH decreases with increasing plant concentration as the DPPH acts as a radical capture (antioxidant) as the plant extract concentration increases the DPPH is consumed more hence the DPPH concentration decreases. Whereas the absorbance of extract at 0.2 ml recorded was 1.8% and for 1 ml the absorbance of extract recorded was 0.4%. Thus the absorbance decreases as the concentration of sample increases. When the reducing power assay was conducted, color change was observed in the sample tubes. It increases with increasing concentration of oil-rich extract obtained from leaves of kale. Thus, if there is more antioxidant compound then more color will be observed in reaction mixture as antioxidant compound forms complex with the components. The difference in behavior of plant extracts may be due to difference in relative abundance of antioxidants agents i.e. proportion of reluctant and oxidants. It may be due to the dilution of reaction system after addition of higher volume of extract.
Conclusion
The yield of essential oil obtained via steam distillation was measured depends on the amount of plant material used. Solvents used for extraction showed rapid physiologic absorption of the extract with low toxicity. High antioxidant potential of Extracts in cell-free systems can either be positive or toxic in the cellular assay, depending on the concentration of extract and conditions of the cell. Kale leaves prove to be a source of bioactive phenolic compounds. Phytochemical screening was done of components from leaves. Medicinally active components obtained from the Brassica oleracea (kale) leaves extract indicate presence of flavonoids, alkaloids, phenolic compounds, tannins, terpenoids, glucosinates, and fatty acids. They proved to exhibit antioxidants properties and therefore can help in prevention of diseases such as hepatotoxicity, neurodegenerative disorders, and chronic diseases. Extracts that run into a spectrophotometer to characterize its wavelength absorption showed best result for ethanol. Moreover, solvent ratio and stock solution volume showed an important influence on the method response
DPPH antioxidant Activity of kale leaves indicates %inhibition as 80% highest obtained from Acetone washing at 0.2 ml concentration. The results obtained from the antioxidant activity and need to be investigated in biological models more closely related to in-vivo solution.
Author’s Contribution
Syeda Hafsa Aamir: Conceived the idea, conducted the research, performed the experiments, collected data and wrote the article.
Aiman Yaseen Butt: Conducted the research, performed the experiments, collected data and wrote the article.
Seema Ashraf: Supervised and designed the study and analyzed the data.
Rashida Ali: Helped insight analysis of data.
References
- Andrade BFN, Barbosa LN, Probst IS, Fernandes J (2013) Antimicrobial activity of essential oils. J Essen Oil Res 26: 34-40.
- Bajpai VK, Baek, Kwang-Hyun (2016) Biological efficacy and application of oils in foods-A Review. AGRIS 19: 1-19.
- Marecik BM, Kubzdela ER, Marecik R (2017) Characterization of phenolics, glucosinolates and antioxidant activity of beverages based on apple juice with addition of frozen and freeze-dried curly kale leaves (Brassica oleracea L. var. acephala L.). Food Chemistry 230: 271-280.
- Murador DC, Mercadante AZ, Ross VV (2016) Cooking techniques improve the levels of bioactive compounds and antioxidant activity in kale and red cabbage. Food Chemistry 196: 1101-1107.
- Bo Sun, Huizhuan Yan, Fen Zhang, QiaomeiWang (2012) Effects of plant hormones on main health-promoting compounds and antioxidant capacity of Chinese kale. Food Res Int 48: 359-366.
- Dhifi W, Bellili S, Jazi S, Bahloul N, Mnif W (2016) Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines (Basel) 3: e25.
- Kumar S, BS Bajwa, Singh K, Kalia AN (2013) Anti-inflammatory activity of herbal plants: A review. IJAPBC 2: 272-273.
- Chenard CA, Zimmerman B, Karen L, Patricia F, Terry L (2015) New measured weight for one cup raw kale reduces nutrient Intake of individuals following the Wahls™ diet. Article Procedia Food Science 4: 39-47.
- Ayaz FA, Ayaz SH, Karaoglu SA, Grúz J, Valentová K, et al. (2008) Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chemistry 107: 19-25.
- Seif HS (2016) Physiological changes due to hepatotoxicity and the protective role of some medicinal plants. J Appl Sci 5: 134-146.
- Wiseman H (2005) Phytochemicals epidemiological factors. Encyclopedia of Human Nutrition (Second Edition) 497-509.
- Björkman M, Klingen I, Birch A, Bones A, Bruce TJ, et al. (2011) Phytochemicals of Brassicaceae in plant protection and human health-Influences of climate, environment and agronomic practice. Phytochemistry 72: 538-556.
- Howard L (2008) Processing techniques and their effect on fruit and vegetable phytochemicals. improving the Health-Promoting properties of fruit and vegetable products, 449-447.
- Katsunori Sasaki, Makiko Neyazaki, Kazutoshi Shindo, Toshiya Ogawa, Masaki Momose (2012) Quantitative profiling of glucosinolates by LC-MS analysis reveals several cultivars of cabbage and kale as promising sources of sulforaphane. J Chromatography B 903: 171-176.
- Kumar R, Tripathi YC (2011) Natural dyes from forest biomass. Forest Research Institute.
- Fadigas JC, Ana MP, Jesus R, Lima D, Wallace D, (2016) Use of multivariate analysis techniques for the characterization of analytical results for the determination of the mineral composition of kale. Microchemical J 96: 352-356.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences