Glucosinolates from broccoli: Nutraceutical properties and their purification
Daniel Villarreal-García and Daniel A Jacobo-Velázquez
| Daniel Villarreal-García and Daniel A Jacobo-Velázquez* Centro de Biotecnología-FEMSA, School of Engineering and Sciences, Tecnológico de Monterrey, Campus Monterrey, Monterrey, N.L. México |
| *Corresponding Author: Jacobo-Velázquez, Centro de Biotecnología-FEMSA, School of Engineering and Sciences, Tecnológico de Monterrey, Campus Monterrey, Monterrey, N.L. México. Tel: 528183582000; Fax: +52-818-328-4136; E-mail: djacobov@itesm.mx |
| Received: September 03, 2016, Accepted: March 07, 2016, Published: March 10, 2016 |
Visit for more related articles at Journal of Nutraceuticals and Food Science
Introduction |
||
| Broccoli (Brassica oleracea var. italica) is a plant from the Brassicaceae family. It has been stated that plants from the Brassica genus have been cultivated for over 2,500 years, though the varieties of broccoli that we know today may have been developed from selections made in Italy in the last 2,000 years [1]. | ||
| Broccoli is a very important crop in economic terms. Worldwide, over 21 million tons of broccoli are produced each year [2]. However, poor postharvest practices cause deterioration in the visual quality of plant foods, making them unsuitable for consumption. It is estimated that up to 35% of the horticultural crops grown are lost due to inadequate postharvest practices. However, these wastes still represent a good source of nutraceutical compounds that can further be subjected to isolation processes and, due to the significant economic loss that they represent for farmers, it is important to find an alternative use for this lost crops [3,4]. Finding alternative uses to broccoli can represent an advantage for farmers, marketers, and even consumers, and can also be an interesting opportunity to create a positive social and economical impact. | ||
| Nutraceuticals are compounds naturally present in fruits and vegetables and can also be obtained at optimum concentrations from dietary supplements [5]. In certain countries, there is awareness of the health-benefits of nutraceuticals. | ||
| Therefore, the global nutraceutical market has an accelerated growth, being of 140 billion dollars with an estimated growth rate of 14.7% [6]. Broccoli is an excellent source of nutraceuticals, and thus, when not acceptable for human consumption it could be used as raw material for the extraction and purification of nutraceuticals for their subsequent use in the dietary supplements industry. | ||
Bioactive Compounds in Broccoli |
||
| Broccoli is an excellent source of bioactive compounds, and thus, it is considered a functional food. Phenolic compounds are among the nutraceuticals present in broccoli, mainly hydroxycinnamic acids and flavonoids [7]. It has been shown that these compounds possess a great antioxidant activity and may play a role in the prevention of several diseases, such as diabetes [8], cardiovascular diseases [9], and neurodegenerative diseases [10,11]. | ||
| Additionally, broccoli contains high concentrations of vitamin C, an essential nutrient. Vitamin C is a hydrosoluble vitamin necessary for a normal development and it is used for wound healing, repairing of cartilage and bones, and collagen synthesis, an essential protein found on skin and ligaments [12]. Moreover, vitamin C may act as a potent antioxidant, helping to prevent atherosclerosis and cancer [13]. | ||
| Carotenoids can also be found in broccoli [14,15]. Lutein, a carotenoid of the xanthophylls group, is one of the most abundant carotenoids present in broccoli, is present throughout the retina, and it has been reported that it protects the eye by filtering hazardous light, preventing agerelated macular degeneration and cataracts [16]. Besides, previous studies have shown that lutein exhibits chemoprotective activity against different types of cancer [17-20]. | ||
| Reports suggest that xanthophylls exert their anticarcinogenic activity through several mechanisms, among which selective induction of apoptosis, inhibition of angiogenesis, and prevention of oxidative damage stand out [21]. | ||
| There is also growing evidence that suggests that the ingestion of lutein may prevent heart disease and stroke, given that previous studies show that lutein reduces the oxidation of low density lipoproteins [22,23] and epidemiological studies indicate an inverse relation between circulating xanthophylls and artery thickness [24,25]. | ||
| Meanwhile, glucosinolates are among the most distinctive nutraceutical compounds in cruciferous vegetables, including broccoli. Their high potential to prevent chronic diseases, especially cancer, has made them subject to thorough research. Therefore, this article is mainly focused on glucosinolates. | ||
Health benefits of glucosinolates and isothiocyanates |
||
| Glucosinolates are plant secondary metabolites that are present in different concentrations among plant organs and throughout the developmental stages of the plant. Moreover, it is thought that glucosinolates, together with myrosinase, are part of a defense mechanism implemented by plants to protect themselves against biotic and abiotic stress [26]. | ||
| Glucosinolates are the compounds that attract the most attention in broccoli. It has been reported that broccoli, through glucosinolates, may act against different types of cancer [27]. Yet, the nutraceutical properties of glucosinolates may be attributed to isothiocyanates, their hydrolysis products [28]. Isothiocyanates are unstable and reactive compounds with the chemical structure R-N=C=S and possess great anticarcinogenic activity, which they achieve through different molecular mechanisms. | ||
Clinical evidence on isothiocyanate anticarcinogenic potential |
||
| Clinical studies designed to measure the anticarcinogenic activity of isothiocyanates have been done. Hecht et al [29] found that phenetyl isothiocyanate (PEITC), produced from the glucosinolate gluconasturtiin, inhibits the metabolic activation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK). In this study, eleven smokers avoided any source of isothiocyanates. Urine samples were taken every 24 h. After three days, they consumed 19-38 mg/day of PEITC for another three days and urine samples were taken every 24 h. A follow-up period was made, were urine samples were taken every 24 h after one or two weeks of PEITC consumption. Results showed that detoxification products of NNK increased after PEITC consumption and returned to baseline levels in the follow-up period, suggesting that PEITC inhibits the oxidative metabolism of NNK. | ||
| Shapiro et al [30] performed a phase I clinical trial on healthy volunteers using a glucosinolate mixture primarily consisting of glucoraphanin (GPN) and an isothiocyanate extract containing mainly sulforaphane (SFN) to evaluate their anticarcinogenic potential. Subjects were divided into three cohorts, each one with different doses: 25 μmol of glucosinolates (group A), 100 μmol of glucosinolates (group B), and 25 μmol of isothiocyanates (group C). Mean excretion levels of dithiocarbamates (isothiocyanates excretion products) in groups A and B were very similar (~18 ± 8.6% and ~20 ± 11.7% of dose, respectively) and much higher and consistent in group C (~71 ± 2% of dose), indicating a low and varying absorption rate of glucosinolates in human intestine. Besides, toxicity tests revealed that no significant adverse effects in liver or thyroid could be attributed to the extracts, implying glucosinolate and isothiocyanate biosafety at the tested doses. | ||
Mechanisms of action |
||
| Induction of phase II enzymes: Sulforaphane has been one the most studied isothiocyanates and has been shown to be very effective in preventing cancer. One of the mechanisms by which isothiocyanates may prevent cancer is by selectively inducing the transcription of phase II enzymes, which play an important role in xenobiotic detoxification [31,32]. Stimulation of transcription of phase II enzyme genes is achieved through antioxidant/electrophile response elements present in their promoter regions. Specifically, isothiocyanates bind to cysteine residues of the protein Keap1, which interacts with the transcription factor Nrf2. This releases Nrf2 from Keap1, which allows its translocation into the nucleus, where it interacts with the mentioned antioxidant response elements to induce the synthesis of phase II enzymes, such as quinone reductase and glutathione-S-transferases [33]. | ||
| Phase II enzymes mainly catalyze reactions such as methylation, sulfation, acetylation, and glutathione conjugation to produce mostly molecules with greater hydrosolubility. This gives these enzymes the ability to inactivate pharmacologically active compounds and to biotransform endogenous substances and xenobiotics, like carcinogens and mutagens, into more excretable forms [34]. Thus, the activation of phase II enzymes by isothiocyanates can be a potent anticancer mechanism. | ||
| Inhibition of phase I enzymes: Other mechanism followed by isothiocyanates to prevent carcinogenesis is through the inhibition of phase I enzymes. Cytochrome P-450 (CYP) enzymes are a type of phase I enzymes, and, besides playing a role in xenobiotic metabolism like phase II enzymes, they also have the ability to transform and activate procarcinogens that may later induce mutations [35]. Nevertheless, previous studies show that isothiocyanates are capable of inhibiting them [36]. Moreover, it has been reported that isothiocyanates with longer alkyl chain length have greater inhibitory activity [37]. However, this ability to inhibit phase I enzymes may depend to a great extent on the isothiocyanate structure, as well as on the tissue or the carcinogenic agent [35]. | ||
| Inflammation and apoptosis: Additionally, isothiocyanates have shown to mediate antitumor activities by inhibiting the growth of cancer cells, including prostate, breast, lung, cervical, and colorectal cancers [35]. An association between cancer and inflammation has been thoroughly studied. Epidemiologic studies have related the predisposition for cancer development with inflammation [38]. Isothiocyanates are capable of suppressing the activity of the nuclear factor kappa B (NF-κB), which induces the expression of proinflammatory genes (i.e. cytokines and chemokines) through the inhibition of the oncoprotein Akt1 [35]. Furthermore, NF-κB is activated by oxidative stress, which may also be attenuated by isothiocyanates [39]. | ||
| Likewise, isothiocyanates present in broccoli are capable of inducing apoptosis and cell cycle arrest. Apoptosis progression is, to a great extent, regulated by the caspase pathway. It has been reported that treatment of cells with isothiocyanates induces the activation of caspase pathway, leading to apoptosis [35]. Additionally, cell cycle is also affected by isothiocyanate treatment. Previous studies showed an accumulation of cells in G2/M phase 16 hours after treatment, which derived in a decline on the growth rate of cells [40], and an induction in the expression of protein p21, which regulates phase S in the cell cycle [41]. It was also demonstrated that isothiocyanates are capable of decreasing the formation of DNA adducts [42,43]. | ||
Dietary Supplements and Biotechnological Production |
||
| Glucosinolates may be found in the dietary supplements market in several forms, such as extracts of broccoli and other cruciferous vegetables or as broccoli sprouts rich in glucoraphanin [44]. However, broccoli extracts may not be as effective because glucosinolates have to be hydrolyzed in order to be completely absorbed in the gastrointestinal tract, though some of them can be hydrolyzed by the gut microflora [45]. Nevertheless, products such as EnduraCell have preserved the activity of myrosinase in order to yield isothiocyanates in an effective manner. | ||
| Regarding their biotechnological production, the most common way of producing glucosinolate dietary supplements is by using plants of the Brassica genus. Black kale was recently identified as one of the greatest source of glucosinolates [46,47], though broccoli is often used to produce extracts for the dietary supplements industry. Nevertheless, it is important to notice that glucosinolates may vary depending on the organ of the plant. For example, it has been reported that aliphatic glucosinolates may be found in greater proportions in florets and leaves, while indolyl glucosinolates are likely to be predominant in roots [48]. | ||
| Additionally, it has been found that total glucosinolate content varies depending on the developmental stage of the plant, for example, it has been reported that the concentration of glucosinolates is higher in broccoli sprouts than in seeds [49]. Moreover, glucosinolate content may vary greatly among different cultivars [50]. Glucosinolates found in broccoli are shown in Table 1: | ||
Glucosinolate isolation for analytical purposes |
||
| There are several methodologies for glucosinolate extraction reported in the literature. However, most of them are aimed towards quantitative or qualitative analyses of plant foods. For example, Truscott and Minchinton [53] report a method for the isolation of glucosinolates from cruciferous plants, where an ion-exchange chromatography procedure is followed, however, desulphation of glucosinolates is done for further analysis by high-performance liquid chromatography, hence, these isolates would not be useful for the production of glucosinolate-based dietary supplements given that intact glucosinolates are needed in order to yield bioactive isothiocyanates. | ||
| Methanol and water are often used as extraction solvents for the purification of intact glucosinolates. Renuka and Thangam [53] use 80% methanol to extract glucosinolates from Brassica oleracea var rubra, however, the use of cold methanol may not yield the highest possible concentration of glucosinolates. Therefore, the use of hot methanol is preferred. | ||
Production of dietary supplements |
||
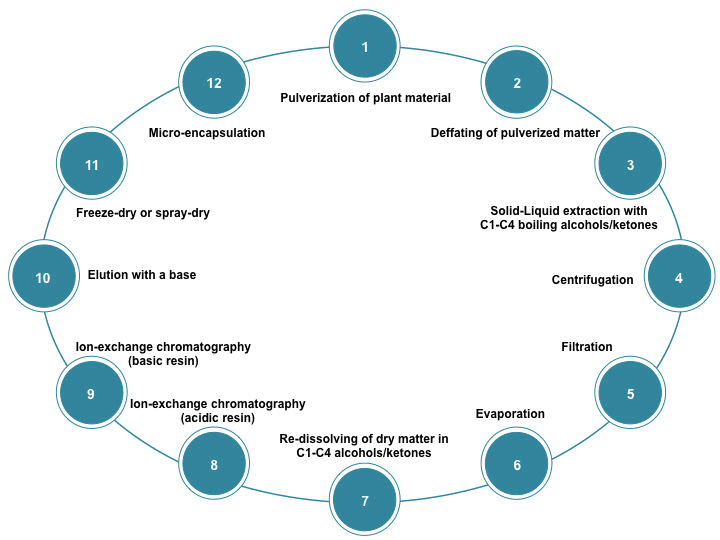
| US patent number US20130030162 A1 [54] proposes a process for the production of a glucosinolate-containing extract (Figure 1) | ||
| The procedure starts with the defatting of the plant material (i.e. broccoli florets, sprouts, or seeds), is recommended to be pulverized in order to achieve a better yield in the extraction process. Defatting may be done by adding hexane and agitating for 3 hours. Solvents used for extraction may be C1- C4 alcohols (e.g. ethanol) or C3-C4 ketones (e.g. acetone), or mixtures of both in an aqueous medium. | ||
| As stated before, broccoli that is not suitable for human consumption due to inadequate postharvest practices may represent a rich source of glucosinolates. Most of the glucosinolates in these lost crops are well preserved as a result of the cell compartmentalization, which keeps myrosinase apart from their substrate [52], however, cell rupture is likely to occur in the extraction process, therefore, it is important to inactivate the enzyme in order to preserve glucosinolate content during the whole process, which may be done by performing the extraction at temperatures around 70ºC. The mixture must be centrifuged in order to obtain an alcoholic/ ketonic extract, which may be filtered to separate insoluble matter from the mixture and evaporated to remove volatiles. If evaporated, it must be re-dissolved in water, alcohols, ketones, or a mixture thereof. For a greater purification, the extract may be subjected into a cation-exchange column in its acidic form that meets regulatory requirements for food processing. The extract will now be adsorbed onto a basic resin suitable from a regulatory point of view. The elution of glucosinolates form the column can be done with a base such as sodium hydroxide, ammonia dissolved in water, an alcohol, or a mixture. The eluate may be then evaporated, freeze-dried, or spray-dried to yield a solid extract, which will be rich in glucosinolates. The obtained solid powder may be further subjected to microencapsulation. | ||
| Other reported methods include the use of more complex extraction solvent systems. For example, Fahey et al. [55] proposed separation and purification by high-speed countercurrent chromatography using a highly polar mixture of 1- propanol-acetonitrile-ammonium sulfate-water (1:0.5:1.2:1) on semi-preparative scale to purify glucosinolates from a variety of plant sources before transferring into preparative scale. the authors report >95% of purity of glucosinolates from broccoli seed extract. | ||
| Finally, as stated before, broccoli that do not meet quality standards still represent a good source of glucosinolates, and when subjected to extreme postharvest abiotic stress conditions could be converted into biofactories of nutraceuticals [3]. For example, Villarreal-García et al. [56] reported the accumulation of high-value glucosinolates after treatment of whole and wounded samples with phytohormones (ethylene and methyl jasmonate). Hence, the stressed broccoli tissue could be subjected to procedures as those described before to extract and purify the high-value glucosinolates accumulated. | ||
Conclusions |
||
| Broccoli is a rich source of bioactive compounds, such as carotenoids, phenolic compounds, vitamin C, and glucosinolates. Regarding glucosinoaltes, several in vitro, in vivo and clinical studies have demonstrated the potential of their hydrolysis products to prevent cancer. Additionally, a substantial amount of broccoli is lost due to poor postharvest practices, however, this plant material not meeting quality standards for human consumption still has important concentrations of bioactive compounds. Hence, broccoli considered as waste may be attractive as raw material for the extraction and purification of glucosinolates, which may be further used in the pharmaceutical and dietary supplements industries. | ||
Acknowledgement |
||
| This study is based upon research supported by research funds from Consejo Nacional de Ciencia y Tecnología (CONACYT, México) Grant (177012) and Tecnologico de Monterrey (Bioprocess and Synthetic biology Research Group). Author D.V.-G. also acknowledges the scholarship (296572) from CONACYT. | ||
Tables at a glance |
||
|
||
Figures at a glance |
||
|
||
References |
||
|
Select your language of interest to view the total content in your interested language
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences