Vitamin B12, A Natural and Green Catalyst for the One-pot Three-Component Synthesis of 4H-Pyran Annulated Systems
Mohammad Dodangeh, Malek-Taher Maghsoodlou, Mehrnoosh Kangani, Farideh Paymozd and Nourallah Hazeri
Mohammad Dodangeh, Malek-Taher Maghsoodlou*, Mehrnoosh Kangani, Farideh Paymozd and Nourallah Hazeri
Department of Chemistry, Faculty of Science, University of Sistan and Baluchestan, Zahedan, Iran
- *Corresponding Author:
- Malek-Taher Maghsoodlou
Department of Chemistry
Faculty of Science
University of Sistan and Baluchestan
P. O. Box: 98135-674, Zahedan
Iran
Tel: +985412416586
Fax: +985412416586
E-mail: mt_maghsoodlou@yahoo.com
Received date: April 25, 2016; Accepted date: July 27, 2016; Published date: July 29, 2016
Citation: Dodangeh M, Maghsoodlou MT, Kangani M et al. Vitamin B12, A Natural and Green Catalyst for the One-pot Three-Component Synthesis of 4H-Pyran Annulated Systems. Curr Trends Nutraceuticals. 2016, 1:2.
Abstract
Vitamin B12 was found as a natural and efficient catalyst for the one-pot three-component synthesis of 4H-pyran annulated systems from the condensation between aryl aldehydes, malononitril and 1,3 dicarbonil compounds in aqueous media at ambient and thermal condition. Vitamin B12 is an organometallic compound that can play the catalytic role in the organic reactions. It has many active sites that make this catalyst affect significantly in spite of its very low amount (0.00017 g). This methodology has number of advantages such as: use of very small amount of catalyst, easy access, short reaction times, high yields, easy work up and use of non-toxic and hazardous catalyst and solvents. Although, all products were obtained just with a simple filtration and no need to column chromatography.
Keywords
Vitamin B12; 4H-pyrane annulated systems; High yields; Non-toxic; Hazardous catalyst; Solvents
Introduction
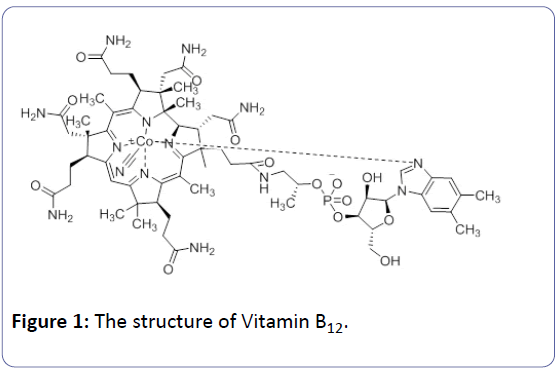
The word “Vitamin B12” is generally used for cyanocobalamin that is a water-soluble substance. Vitamin B12 is the first organometallic compound that naturally occurs. In addition, it is a crucial nutrient for human growth and cell development [1]. Vitamin B12 (molecular weight 1355.4) is stable in aqueous solution between pH 4 and 7 can be heated at 120ÃÆââ¬Â¹Ãâà ¡C without significant loss [2]. The B12 not only has biological function in nervous system, but also diminishes the risk of heart diseases. This vitamin plays an essential role in human biological system, include DNA synthesis and regulation, enzymatic reaction, red blood cell formation and etc. [3]. Moreover, It has a complex organometallic cofactor with a central cobalt (III) atom to coordinate with coring ring consisting of six donor ligands [3,4] (Figure 1). The performed studies about the vitamin B12’s structure and biochemistry in the areas of chemistry, psychology and medicine have awarded four Noble [4]. Vitamin B12 has been already used for many organic reactions such as: methyltransferases [5], the asymmetric catalyst for the enantioselective cyclopropanation of alkenes [6], catalyzed carbon-carbon bond forming reaction [7] and catalyzed dehydrogenation reaction [8].
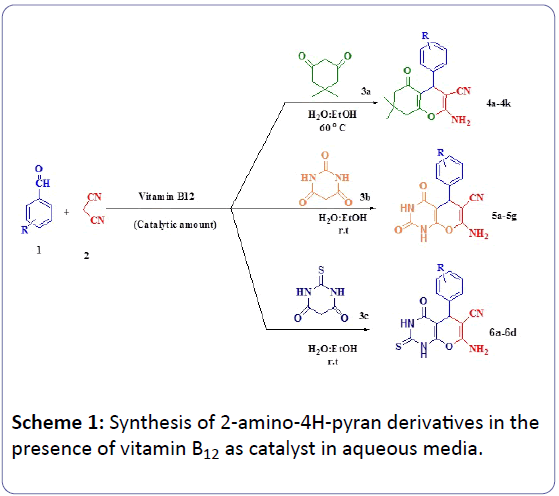
Tetrahydrobenzopyran, and their derivatives are of considerable interest as they includes a wide range of biological properties [9], such as spasmolytic, diuretic, anticoagulant, anticancer, and anti-anaphylactic activity [10]. In addition, they can be used as cognitive enhancers for the treatment of neurodegenerative diseases, containing amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, AIDS-associated dementia, Alzheimer’s disease, and Down’s syndrome, as well as for the treatment of schizophrenia and myoclonus [11]. In addition, a number of 2- amino-4H-pyrans are useful as photoactive materials [12]. These molecules are biologically active and find application in pharmacological properties such as anticoagulant, spasmolytic, diuretic, anti-anaphylactic, and anticancer agents [10]. Some of the 2-aminobenzochromene derivatives are useful cosmetics and pigments [13] and are utilized as potential biodegradable agrochemicals [14]. Some methods have been reported for the preparing of tetrahydrobenzopyran, and pyrano [2,3-d] pyrimidine derivatives [15-29]. However, some of these methods have drawbacks, such as long reaction times, use of expensive reagents, low yields, harsh reaction conditions, effluent pollution, and tedious work-up procedures. In continue of our research on multi-component reactions [30-37], herein we report easy and green synthesis of 4H-pyran annulated systems from the reaction between aromatic aldehydes, malononitrile and dimedone/barbituric acid and thiobarbituric acid in the presence of vitamin B12 as catalyst in aqueous media at ambient and thermal conditions (Scheme 1).
Methodology
Melting points and IR spectra were measured with an Electro-thermal 9100 apparatus and a JASCO FT-IR-460 plus spectrometer, respectively. The 1H NMR spectra were obtained on Bruker DRX-400 & 300 Advance instruments with DMSO as a solvent. All reagents and solvents are obtained from Fluka and Merck and used without further purification. The Vitamin B12 was purchased from the Sigma-Aldrich company. TLC was performed on Silica–gel Polygram SILG/UV 254 plates.
General procedure for the synthesis of 2- amino-7,7-dimethyl-5-oxo-5,6,7,8- tetrahydro-4H-chromenes
Vitamin B12 (0.00017 g) dissolved in H2O: Et-OH (3:1 mL), then a mixture of aromatic aldehyde (1 mmol), malononitrile (1 mmol), and 1,3-dicarbonyl compounds (1 mmol) was added to above solution and stirred at 60°C for products (4a-4h) and ambient temperature for other products (5a-5g, 6a-6d). After completion of the reaction, as indicated by thin-layer chromatography (TLC), the reaction mixture was filtered and residue was washed with ethanol (3 × 5 mL) to separate catalyst. The crude product was recrystallized from ethanol to afford the pure product. The desired pure products were characterized by comparison of their physical data (melting points, IR and 1H NMR) with those of known compounds in the literature.
Spectral data for selected products
2-Amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro- 4H-chromene-3-carbonitrile (4b).
IR (KBr, cm-): 3329, 3394,3215, 2204, 1681; 1H NMR (400 MHz, DMSO-d6): δ (ppm): 1.02 (s, 3H),1.14 (s,3H), 2.13 (d, J = 16.1 Hz, 1H), 2.28 (d, J = 16.2 Hz, 1H),2.56 (s, 2H),4.30 (s, 1H), 6.25 (s, 2H, br), 7.17–7.32 (5H, Ar).
2-Amino-5,6,7,8-tetrahydro-4-(4-methyl)-7,7-dimethyl-5- oxo-4H-chromene-3- carbonitrile (4f)
IR (KBr, cm-): 3466, 3322, 2954, 2191, 1675, 1248; 1H NMR (400 MHz, DMSO-d6): δ (ppm): 1.09 (s, 3H), 1.12 (s, 3H), 2.22 (dd, J = 16.4 Hz, 2H), 2.31 (s, 3H), 2.46 (dd, J = 17.6, 2H), 4.53(s, 2H), 4.71(s, 1H), 6.713–6.808 (m, 2H), 6.971 (t, 1H).
2-Amino-5,6,7,8-tetrahydro-4-(4-hydroxyphenyl)-7,7- dimethyl-5-oxo-4Hchromene-3-carbonitrile (4h)
IR (KBr, cm-): 3,287, 3,165, 2,962, 2,184, 1,672, 1,208; 1H NMR (400 MHz, DMSO-d6): δ (ppm): 1.051(s, 3H), 1.11 (s, 3H), 2.26 (dd, J = 16.4 Hz, 2H), 2.465 (s, 2H), 4.34 (s, 1H), 4.51(s, 2H), 5.24 (s, 1H), 6.725–7.104 (dd, J = 8.4, 4H).
IR (KBr, cm-): 3,287, 3,165, 2,962, 2,184, 1,672, 1,208; 1H NMR (400 MHz, DMSO-d6): δ (ppm): 1.051(s, 3H), 1.11 (s, 3H), 2.26 (dd, J = 16.4 Hz, 2H), 2.465 (s, 2H), 4.34 (s, 1H), 4.51(s, 2H), 5.24 (s, 1H), 6.725–7.104 (dd, J = 8.4, 4H).
3-carbonitrile (4j)
IR (KBr, cm-1): 3,304, 3,205, 2,947, 2,172, 1,674, 1,213; 1H NMR (400 MHz, DMSO-d6): δ (ppm): 1.07 (s, 3H), 1.13 (s, 3H), 2.24 (dd, J = 16 Hz, 2H), 2.44 (dd, J = 17.6, 2H), 3.83 (s, 3H), 3.94 (s, 3H), 4.52(s, 2H), 4.77(s, 1H), 6.712–6.807 (dd, J = 8, 2H), 6.973 (t, J = 8, 1H).
7-amino-5-(4-nitrophenyl)-2,3,4,5-tetrahydro-2,4-dioxo-1Hpyrano[ 2,3-d]pyrimidine-6-carbonitrile (5a)
1H NMR (300 MHz, DMSO-d6): δ (ppm): 4.43 (s, 1H, CH), 7.30-8.45 (m, 6H, Ar & NH2), 11.15 (s, 1H, NH), 12.20 (s, 1H, NH).
7-amino-5-(4-bromophenyl)-2,3,4,5-tetrahydro-2,4- dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (5b)
1H NMR (300 MHz, DMSO-d6): δ (ppm): 4.24 (s, 1H, CH), 7.18-7.85 (m, 6H, Ar & NH2), 11.14 (s, 1H, NH), 12.12 (s, 1H, NH).
7-amino-5-(4-chlorophenyl)-2,3,4,5-tetrahydro-2,4- dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (5d)
1H NMR (300 MHz, DMSO-d6): δ (ppm): 4.25 (s, 1H, CH), 7.18 (s, 2H, NH2), 7.24-7.34 (m, 4H, Ar), 11.11 (s, 1H, NH), 12.11 (s, 1H, NH).
7-amino-5-(4-fluorophenyl)-2,3,4,5-tetrahydro-2,4- dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (5e)
1H NMR (300 MHz, DMSO-d6): δ (ppm): 4.25 (s, 1H, CH), 7.08-7.16 (m, 6H, Ar & NH2), 11.01 (s, 1H, NH), 12.10 (s, 1H, NH).
7-amino-5-(3-chlorophenyl)-2,3,4,5-tetrahydro-2,4-dioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (5g)
1H NMR (300 MHz, DMSO-d6): δ (ppm): 4.27 (s, 1H, CH), 7.18-7.34 (m, 6H, Ar & NH2), 11.10 (s, 1H, NH), 12.30 (s, 1H, NH).
7-amino-5-(4-bromophenyl)-2,3,4,5-tetrahydro-4-oxo-2- thioxo-1H-pyrano[2,3-d]pyrimidine-6-carbonitrile (6b)
1H NMR (300 MHz, DMSO-d6): δ(ppm): 4.93 (s, 1H, CH), 5.91 (s, 2H, NH2), 6.94-7.37 (m, 4H, Ar), 11.66 (s, 1H, NH), 12.26 (s, 1H, NH).
Results and Discussions
In order to be able to synthesis of 2-amino-4H-pyran derivatives in a more efficient way, minimizing the time and amount of catalyst, the reaction of 4-nitrobenzaldehyde, malononitrile and dimedone/barbituric acid/thiobarbituric acid was selected as a model system. The reaction was carried out in different solvents, and temperatures. The best results were obtain in H2O: Et-OH (3:1 mL) at ambient temperature for the 5a and 6a and 60°C for the 4a (Table 1). As can be seen in Table 2, 12.54 μmol %) of vitamin B12 (0.00017 g) was the most effective amount to catalyze the reactions.
| Entry | product | Solvent | Catalyst | Temperature | Isolated yield % |
|---|---|---|---|---|---|
| 1 | 4a | H2O | Vitamin B12 | 60 | 70 |
| 2 | 4a | H2O:EtOH (2:1) | Vitamin B12 | 60 | 71 |
| 3 | 4a | H2O:EtOH (3:1) | Vitamin B12 | 60 | 86 |
| 4 | 4a | H2O:EtOH (4:1) | Vitamin B12 | 60 | 65 |
| 5 | 4a | H2O:EtOH (3:1) | Vitamin B12 | r.t | - |
| 6 | 4a | H2O:EtOH (3:1) | Vitamin B12 | 40 | 45 |
| 7 | 4a | H2O:EtOH (3:1) | Vitamin B12 | 50 | 70 |
| 8 | 5a | H2O:EtOH (3:1) | Vitamin B12 | r.t | 94 |
| 9 | 5a | H2O:EtOH (3:1) | Vitamin B12 | 50 | 60 |
| 10 | 5a | H2O:EtOH (1:1) | Vitamin B12 | r.t | 65 |
| 11 | 6a | H2O:EtOH (3:1) | Vitamin B12 | r.t | 80 |
| 12 | 6a | H2O:EtOH (3:1) | Vitamin B12 | 50 | 66 |
| 13 | 6a | H2O:EtOH (1:1) | Vitamin B12 | r.t | 70 |
Table 1: Optimization of solvent and temperature in synthesis of compound 4a, 5a and 6a.
| Entry | product | Catalyst µ (mol %) | Time (min) | Yield(%) |
|---|---|---|---|---|
| 1 | 4a | 2.21 | 80 | 60 |
| 2 | 4a | 3.7 | 35 | 71 |
| 3 | 4a | 8.12 | 29 | 82 |
| 4 | 4a | 12.54 | 15 | 86 |
| 5 | 4a | 15.5 | 20 | 86 |
| 6 | 4a | 70.38 | 20 | 86 |
| 7 | 5a | 2.21 | 15 | 70 |
| 8 | 5a | 3.7 | 13 | 76 |
| 9 | 5a | 8.12 | 10 | 80 |
| 10 | 5a | 12.54 | 4 | 94 |
| 11 | 5a | 15.5 | 5 | 94 |
| 12 | 5a | 70.38 | 5 | 94 |
| 13 | 6a | 2.21 | 15 | 68 |
| 14 | 6a | 3.7 | 10 | 70 |
| 15 | 6a | 8.12 | 8 | 75 |
| 16 | 6a | 12.54 | 5 | 80 |
| 17 | 6a | 15.5 | 5 | 80 |
| 18 | 6a | 70.38 | 5 | 80 |
Table 2: Optimization catalyst in synthesis of compound 4a, 5a and 6a.
Using these optimized reaction, the scope and efficiency of the reaction were explored for the synthesis of a wide variety of 4H-pyrans annulated systems using aromatic aldehydes, malononitriles and 1,3-dicarbonyl compounds. The results are summarized in Table 3 [38-50].
| Entry | Ar | 1,3-dicarbonil compounds | Product | Time (min) | Yeild% | MP(Obs) (°C) | MP(Lit) (°C) [ref] |
|---|---|---|---|---|---|---|---|
| 1 | 4-NO2 C6H4 | 3a | 4a | 15 | 86 | 180-181 | 183-185 [38] |
| 2 | C6H5 | 3a | 4b | 20 | 85 | 229-230 | 233-235 [38] |
| 3 | 4-Cl C6H4 | 3a | 4c | 26 | 79 | 219-220 | 218 [38] |
| 4 | 4-F C6H4 | 3a | 4d | 39 | 80 | 205-206 | 208-210 [39] |
| 5 | 4-Br C6H4 | 3a | 4e | 18 | 75 | 207-208 | 207-209 [40] |
| 6 | 4-Me C6H4 | 3a | 4f | 20 | 85 | 214-215 | 215-218 [41] |
| 7 | 2-NO2 C6H4 | 3a | 4g | 19 | 85 | 221-222 | 223-225 [42] |
| 8 | 4-OH C6H4 | 3a | 4h | 30 | 85 | 212-213 | 214-215 [43] |
| 9 | 3-NO2 C6H4 | 3a | 4i | 120 | 88 | 208-209 | 208-211 [44] |
| 10 | 2,3-(OMe)2 C6H4 | 3a | 4j | 42 | 88 | 217-218 | 214-216 [45] |
| 11 | 3-Cl C6H4 | 3a | 4k | 53 | 82 | 221-222 | 226-227 [36] |
| 12 | 4-NO2 C6H4 | 3b | 5a | 4 | 94 | 245-246 | 245 [46] |
| 13 | 4-Br C6H4 | 3b | 5b | 3 | 91 | 240-241 | 235–236 [47] |
| 14 | C6H5 | 3b | 5c | 5 | 89 | 220-222 | 223 [48] |
| 15 | 4-Cl C6H4 | 3b | 5d | 3 | 89 | 244-245 | 242-244 [46] |
| 16 | 4-F C6H4 | 3b | 5e | 3 | 96 | 232-233 | 225-226 [46] |
| 17 | 4-Me C6H4 | 3b | 5f | 2 | 90 | 226-227 | 225 [48] |
| 18 | 3-Cl C6H4 | 3b | 5g | 3 | 85 | 237-238 | 240-241 [47] |
| 19 | 4-NO2 C6H4 | 3c | 6a | 5 | 80 | 232-233 | 233–235 [49] |
| 20 | 4-Br C6H4 | 3c | 6b | 5 | 90 | 236-237 | 236 [49] |
| 21 | 3-Cl C6H4 | 3c | 6d | 22 | 85 | 237-238 | 237-238 [49] |
| 22 | 3-NO2 C6H4 | 3c | 6e | 20 | 93 | 238-239 | 235-236 [50] |
Table 3: Preparation of 4H-pyran substitutes.
Interestingly, a variety of aryl aldehydes including electron withdrawing or releasing substituents (ortho-, meta-, and para-substituted) participated well in this reaction and gave the product in good to excellent yield.
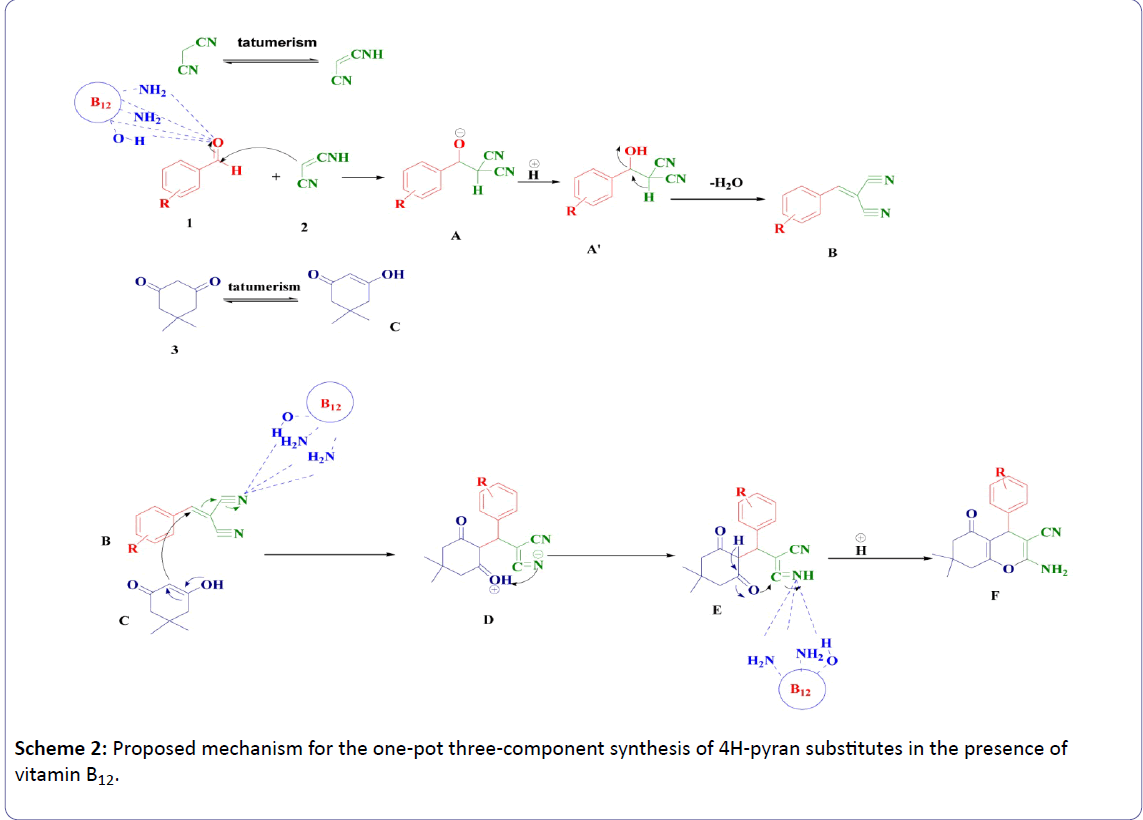
A mechanism was proposed for this reaction. As can be seen in scheme 2, First, Knoevenagal condensation between 1 and 2 produced 2-benzylidenemalononitrile B, Michael addition of B with C (1,3-dicarbonyl compound), and followed cyclization and tautomerization afforded the corresponding product F. There are many reactive sites in the vitamin B12 molecule that can active carbonyl group (Figure 1).
In order to assess the efficiency and generality of this methodology, the obtained result from the reaction of 4- nitrobenzaldehyde and malononitrile with substrate 3a, 3b and 3c by this method has been compared with those of the previously reported methods (Table 4). It was found that the present method is convincingly superior to the reported methods with respect to reaction time, yield of the product and amount of the catalyst.
| Entry | product | Catalyst/Condition | Time | Yield (%) | Reference |
|---|---|---|---|---|---|
| 1 | 4a | Melt/130°C | 1 h | 100 | [38] |
| 2 | 4a | Urea (10 mol%)/ EtOH:H2O/ r.t | 5 h | 92 | [39] |
| 3 | 4a | (NH4)2.HPO4/ H2O/r.t | 2 h | 78 | [40] |
| 4 | 4a | Phenylboronic acid/ EtOH.H2O/reflux | 30 min | 88 | [41] |
| 5 | 4a | Vitamin B12/ H20:EtOH, 60°C | 15 min | 86 | Present work |
| 6 | 5a | Zn|(L) prline|2/ EtOH/reflux | 30min | 90 | [48] |
| 7 | 5a | L-proline/EtOH/r.t | 45 min | 73 | [49] |
| 8 | 5a | Triethanolamine/Choline chloride Zncl2/ 75°C/EtOH | 92 sec | 67 | [50] |
| 9 | 5a | Vitamin B12/H2O:EtOH/r.t | 4 min | 94 | Present work |
| 10 | 6a | L-proline/EtOH/r.t | 90 min | 76 | [49] |
| 11 | 6a | Triethanolamine/Choline chloride Zncl2/ 75°C/EtOH | 240 sec | 42 | [50] |
| 12 | 6a | Vitamin B12/H2O:EtOH/r.t | 5 min | 80 | Present work |
Table 4: Comparison of the efficiency of vitamin B12 with other reported catalysts in literature.
Conclusion
In conclusion, Vitamin B12 can be used as a promising ecofriendly catalyst for the synthesis of 4H-pyrans annulated system. Moreover, this method has several other advantages such as, high yields, operational simplicity, clean and neutral reaction conditions, which makes it a useful and attractive process for the synthesis of a wide variety of biologically active compounds.
Acknowledgment
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
References
- Banerjee R (1999) Chemistry and Biochemistry of B12. John Wiley & Sons.
- Sherma J, Fried B (2003) Handbook of thin-layer chromatography. CRC press 89.
- Giedyk M, Goliszewska K, Gryko D (2015) Vitamin B12catalyzed reactions. ChemSocRev 44: 3391-3404.
- Pozharskii AF, Soldatenkov AT, Katritzky AR (1997) Front Matter. John Wiley & Sons, Ltd.
- Matthews RG (2001) Cobalamin-dependent methyltransferases. Accchem res 34: 681-689.
- Chen Y, Zhang XP (2004) Vitamin B12 derivatives as natural asymmetric catalysts: enantioselectivecyclopropanation of alkenes. J org chem 69: 2431-2435.
- Shey J, McGinley CM, McCauley KM, Dearth AS, Young BT, et al. (2002). Mechanistic investigation of a novel vitamin B12-catalyzedcarbon-carbon bond forming reaction, the reductive dimerization of arylalkenes. Jorgchem 67: 837-846.
- Pratt DA, Van der DWA (2006) On the role of alkylcobalamins in the vitamin B12-catalyzed reductive dehalogenation of perchloroethyleneand trichloroethylene. Chemcomm5: 558-560.
- Shitole NV, Shelke KF, Sadaphal SA, Shingate BB, Shingare MS (2010) PEG-400 remarkably efficient and recyclable media for one-pot synthesis of various 2-amino-4 H-chromenes. Green Chem Letters Rev 3: 83-87.
- Zavar S (2012) A novel three component synthesis of 2-amino-4H-chromenes derivatives using nanoZnO catalyst. Arab J Chem.
- Bonsignore L, Loy G, Secci D, Calignano A (1993) Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur J Med Chem 28: 517-520.
- Konkoy CS, Fick DB, Cai SX, Lan NC, Keana JF, et al.(2004) Substituted 5-oxo-5, 6, 7, 8-tetrahydro-4H-1-benzopyrans and benzothiopyrans and the use thereof as potentiators of AMPA. U.S. Patent 6,680,332.
- Armesto D, Horspool WM, Martin N, Ramos A, Seoane C (1989) Synthesis of cyclobutenes by the novel photochemical ring contraction of 4-substituted 2-amino-3, 5-dicyano-6-phenyl-4H-pyrans. J Org Chem 54: 3069-3072.
- Kumar BS, Srinivasulu N, Udupi RH, Rajitha B, Reddy YT,et al. (2006) An efficient approach towards three component coupling of one pot reaction for synthesis of functionalized benzopyrans. J HeterocyclChem 43: 1691-1693.
- Azath IA, Puthiaraj P, Pitchumani K (2012) One-pot multicomponent solvent-free synthesis of 2-amino-4 H-benzo [b] pyrans catalyzed by per-6-amino-β-cyclodextrin. ACS SusChem& Eng. 1: 174-179.
- Hafez EAA, Elnagdi MH, Elagamey AGA, EL-Taweel FMAA (1987) Nitriles in heterocyclic synthesis: novel synsthesis of benzo [c]-coumarin and of benzo [c] pyrano [3, 2-c] quinoline derivatives. Heterocycles, 26: 903-907.
- Heber D, Heers C, Ravens U (1993) Positive inotropic activity of 5-amino-6-cyano-1, 3-dimethyl-1, 2, 3, 4-tetrahydropyrido [2, 3-d] pyrim idine-2, 4-dione in cardiac muscle from guinea-pig and man. Part 6: Compounds with positive inotropic activity. Die Pharmazie 48: 537-541.
- Grivsky EM, Lee S, Sigel CW, Duch DS, Nichol CA (1980) Synthesis and antitumor activity of 2, 4-diamino-6-(2, 5-dimethoxybenzyl)-5-methylpyrido [2, 3-d] pyrimidine. J Med Chem 23: 327-329.
- Ghorab MM, Hassan AY (1998) Synthesis and antibacterial properties of new dithienyl containing pyran, pyrano [2, 3-b] pyridine, pyrano [2, 3-d] pyrimidine and pyridine derivatives. Phosphorus, Sulfur Silicon Relat. Elem 141: 251-261.
- Devi I, Borah HN, Bhuyan PJ (2004) Studies on uracils: a facile one-pot synthesis of oxazino [4, 5-d]-, pyrano [2, 3-d]-, pyrido [2, 3-d]-and pyrimido [4, 5-d] pyrimidines using microwave irradiation in the solid state. Tetrahedron letters, 45: 2405-2408.
- Davoll, J, Clarke J, Elslager EF (1972). Antimalarial substances. 26. Folate antagonists. 4. Antimalarial and antimetabolite effects of 2, 4-diamino-6-[(benzyl) amino] pyrido [2, 3-d] pyrimidines. J Med Chem 15: 837-839.
- Kretzschmar E (1980) On derivatives of 4-oxo-3, 4-dihydropyrido [2, 3-d] pyrimidine. Pharmazie, 35: 253-256.
- Lian XZ, Huang YQ, Li WJ, ZhengY(2007) MonatshChem 139: 129-131.
- Jin TS, Wang AQ, Shi F, Han LS, Liu LB,et al. (2006) Hexadecyldimethyl benzyl ammonium bromide: an efficient catalystfor a clean one-pot synthesis of tetrahydrobenzopyran derivatives in water. Arkivoc 14: 78-86.
- Devi I, Bhuyan PJ (2004) Sodium bromide catalysed one-pot synthesis of tetrahydrobenzo [b] pyrans via a three-component cyclocondensation under microwave irradiation and solvent free conditions. Tetrahedron Letters, 45(47), 8625-8627.
- Balalaie S, Sheikh-Ahmadi M, Bararjanian M (2007) Tetra-methyl ammonium hydroxide: An efficient and versatile catalyst for the one-pot synthesis of tetrahydrobenzo [b] pyran derivatives in aqueous media. CatalComm 8: 1724-1728.
- Abdolmohammadi S, Balalaie S (2007) Novel and efficient catalysts for the one-pot synthesis of 3, 4-dihydropyrano [c] chromene derivatives in aqueous media. Tetrahedron Letters 48: 3299-3303.
- Davoodnia A, Allameh S, Fazli S, Tavakoli-Hoseini N (2011). One-pot synthesis of 2-amino-3-cyano-4-arylsubstituted tetrahydrobenzo [b] pyranscatalysed by silica gel-supported polyphosphoric acid (PPA-SiO2) as an efficient and reusable catalyst. Chemical Papers 65: 714-720.
- Mobinikhaledi A, Fard MB (2010) Tetrabutylammonium Bromide in Water as a Green Media for the Synthesis of Pyrano [2, 3-d] pyrimidinone and Tetrahydrobenzo [b] pyran Derivatives. ActaChimSlov 57: 931-935.
- Sadeh FN, Maghsoodlou MT, Hazeri N, Kangani M (2015) A facile and efficient synthesis of tetrahydrobenzo [b] pyrans using lactose as a green catalyst. Res ChemIntermed 41: 5907-5914.
- Kangani M, Maghsoodlou MT, Hazeri N (2016) Vitamin B12: An efficient type catalyst for the one-pot synthesis of 3, 4, 5-trisubstituted furan-2 (5H)-ones and N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates. Chin ChemLett27: 66-70.
- Vafajoo Z, Veisi H, Maghsoodlou MT, Ahmadian H (2014) Electrocatalytic multicomponent assembling of aldehydes, 4-hydroxycoumarin and malononitrile: An efficient approach to 2-amino-5-oxo-4, 5-dihydropyrano (3, 2-c) chromene-3-carbonitrile derivatives. ComptesRendusChimie 17: 301-304.
- Adrom B, Maghsoodlou MT, Hazeri N, Lashkari M (2015) Solvent-free synthesis of 1-(benzothiazolylamino) methyl-2-naphthols with maltose as green catalyst. Res ChemIntermed 41: 7553-7560.
- Abadi AYE, Maghsoodlou MT, Heydari R, Mohebat R (2015) An efficient four-component domino protocol for the rapid and green synthesis of functionalized benzo [a] pyrano [2, 3-c] phenazine derivatives using caffeine as a homogeneous catalyst. Res. Chem. Intermed.1-9.
- Sajadikhah SS, Maghsoodlou MT (2014) A simple and green approach for the synthesis of polyfunctionalized mono-and bis-dihydro-2-oxopyrroles catalyzed by trityl chloride. RSC Advances, 4: 43454-43459.
- Hazeri N, Maghsoodlou MT, Mir F, Kangani M, Saravani H, et al. (2014). An efficient one-pot three-component synthesis of tetrahydrobenzo [b] pyran and 3, 4-dihydropyrano [c] chromene derivatives using starch solution as catalyst. Chin J Cata 35: 391-395.
- Hazeri N, Maghsoodlou MT, Mousavi MR, Aboonajmi J, Safarzaei M (2015) Potassium sodium tartrate as a versatile and efficient catalyst for the one-pot synthesis of pyran annulated heterocyclic compounds in aqueous media. Res ChemIntermed 41: 169-174.
- Kaupp G, Naimi-Jamal MR, Schmeyers J (2003) Solvent-free Knoevenagel condensations and Michael additions in the solid state and in the melt with quantitative yield. Tetrahedron 59: 3753-3760.
- Brahmachari G, Banerjee B (2013) Facile and one-pot access to diverse and densely functionalized 2-amino-3-cyano-4 H-pyrans and pyran-annulated heterocyclic scaffolds via an eco-friendly multicomponent reaction at room temperature using urea as a novel organo-catalyst. ACS SusChem&Eng 2: 411-422.
- Balalaie S, Bararjanian M, SheikhÃÆâÃâââ¬ÃâÃÂAhmadi M, Hekmat S, Salehi P (2007) Diammonium Hydrogen Phosphate: An Efficient and Versatile Catalyst for the OneÃÆâÃâââ¬ÃâÃÂPot Synthesis of Tetrahydrobenzo [b] pyran Derivatives in Aqueous Media. Synthetic Commu 37: 1097-1108
- Nemouchi S, Boulcina R, Carboni B, Debache A (2012) Phenylboronic acid as an efficient and convenient catalyst for a three-component synthesis of tetrahydrobenzo [b] pyrans. ComptesRendusChimie 15: 394-397.
- Sarrafi Y, Mehrasbi E, Vahid A, Tajbakhsh M (2012) Well-ordered mesoporous silica nanoparticles as a recoverable catalyst for one-pot multicomponent synthesis of 4H-chromene derivatives. Chin J Catal 33: 1486-1494.
- Katkar SS, Lande MK, Arbad BR, Gaikwad ST (2011) A Recyclable and Highly Effective ZnOÃÆâÃâââ¬ÃâÃÂbeta Zeolite as a Catalyst for OneÃÆâÃâââ¬ÃâÃÂpot ThreeÃÆâÃâââ¬ÃâÃÂComponent Synthesis of Tetrahydrobenzo [b] pyrans. Chin JChem 29: 199-202.
- Jin TS, Wang AQ, Wang X, Zhang JS, Li TS (2004) A clean one-pot synthesis of tetrahydrobenzo [b] pyran derivatives catalyzed by hexadecyltrimethyl ammonium bromide in aqueous media. Synlett 871-873.
- Devi I, Bhuyan PJ (2004) Sodium bromide catalysed one-pot synthesis of tetrahydrobenzo [b] pyrans via a three-component cyclocondensation under microwave irradiation and solvent free conditions. Tetrahedron Letters 45: 8625-8627.
- Yu J, Hanqing W (2005) Green synthesis of pyrano [2, 3ÃÆâÃâââ¬ÃâÃÂd]ÃÆâÃâââ¬ÃâÃÂpyrimidine derivatives in ionic liquids. Synthetic comm 35: 3133-3140.
- Ziarani GM, Faramarzi S, Asadi S, Badiei A, Bazl R, et al. (2013) Three-component synthesis of pyrano [2, 3-d]-pyrimidine dione derivatives facilitated by sulfonic acid nanoporous silica (SBA-Pr-SO 3 H) and their docking and urease inhibitory activity. DARU Journal of Pharmaceutical Sciences 21: 1.
- Heravi MM, Ghods A, Bakhtiari K, Derikvand F (2010) Zn [(L) proline] 2: an efficient catalyst for the synthesis of biologically active pyrano [2, 3-d] pyrimidine derivatives. Synthetic Comms 40: 1927-1931.
- Bararjanian M, Balalaie S, Movassag B, Amani AM (2009) One-pot synthesis of pyrano [2, 3-d] pyrimidinone derivatives catalyzed by L-proline in aqueous media. J Iran ChemSoc 6: 436-442.
- Kumar YD, Quraishi MA (2014) Choline chloride.ZnCl2: green, effective and reusable ionic liquid for synthesis of 7-amino-2, 4-dioxo-5-phenyl-2, 3, 4, 5-tetrahydro-1H-pyrano [2, 3-d] pyrimidine-6-carbonitrile derivative.J Mater Environ Sci 5: 1075-1078.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences