The Potential of Health Supplements to Mitigate the Detrimental Effects of Ionizing Radiation: A Systematic Review
Edward Ashworth1,2,3,4*, Anna Fogtman1,5, Roger Huerta Lluch1,6, Virginia Wotring7, Andrew Winnard4 and Tobias Weber1
1 European Space Agency (ESA), European Astronaut Centre (EAC), Space Medicine Team (HRE-OM), Cologne, Germany 2 Department of Bioengineering, Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand 3 Te P?naha ?tea-Space Institute, University of Auckland, Auckland, New Zealand 4 Space Biomedicine Systematic Review Methods Group, Wylam, UK 5 Space Applications Services NA/SA, Brussels, Belgium 6 KBR GmbH, Cologne, Germany 7 International Space University, Illkirch-Graffenstaden, France
Published Date: 2024-08-14DOI10.36648/ipctn.9.4.45
Edward Ashworth1,2,3,4*, Anna Fogtman1,5, Roger Huerta Lluch1,6, Virginia Wotring7, Andrew Winnard4 and Tobias Weber1
1European Space Agency (ESA), European Astronaut Centre (EAC), Space Medicine Team (HRE-OM), Cologne, Germany
2Department of Bioengineering, Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand
3Te PÅ«naha Ä?tea-Space Institute, University of Auckland, Auckland, New Zealand
4Space Biomedicine Systematic Review Methods Group, Wylam, UK
5Space Applications Services NA/SA, Brussels, Belgium
7International Space University, Illkirch-Graffenstaden, France
- *Corresponding Author:
- Edward Ashworth
Department of Bioengineering, Auckland Bioengineering Institute, University o f Auckland, Auckland,
New Zealand,
E-mail: edward.ashworth@sydney.edu.au
Received date: July 13, 2024, Manuscript No. IPCTN-24-19376; Editor assigned date: July 17, 2024, PreQC No. IPCTN-24-19376 (PQ); Reviewed date: July 31, 2024, QC No. IPCTN-24-19376; Revised date: August 07, 2024, Manuscript No. IPCTN-24-19376 (R); Published date: August 14, 2024, DOI: 10.36648/ipctn.9.4.45
Citation: Ashworth E, Fogtman A, Lluch RH, Wotring V, Winnard A, et al. (2024) The Potential of Health Supplements to Mitigate the Detrimental Effects of Ionizing Radiation: A Systematic Review. J Nutraceuticals Food Sci Vol.9 No.4: 45.
Abstract
The negative effects of ionizing radiation on healthy tissue pose a medical challenge for both human space exploration as well as terrestrial medicine. Therefore, understanding the effectiveness of current treatments is important both in the application of current medicine and the development of new therapeutics. Health supplements are used widely in or by consumers to prevent the occurrence of symptoms following radiotherapy. To evaluate the possible efficacy of health supplements and their potential for use during deep space exploration, a systematic literature review was conducted. A search of PubMed, Cochrane Library and OVID (Medline) databases was conducted, identifying 77 studies that examined therapeutic effects of health supplements on ionizing radiation, of which 15 were eligible for inclusion in the analysis. Health supplements provided no beneficial effects to either functional or biochemical physiological outcomes. However, symptomatic relief was found in a range of irradiated areas of the body from a variety of health supplements following irradiation. The localized actions of both the radiotherapy and the health supplement means further investigations would be required before using these health supplements to protect against whole-body radiation exposure. Further investigations should also focus on the health supplements shown to have the greatest positive effects to address the lack of clinical consensus.
The negative effects of ionizing radiation on healthy tissue pose a medical challenge for both human space exploration as well as terrestrial medicine. Therefore, understanding the effectiveness of current treatments is important both in the application of current medicine and the development of new therapeutics. Health supplements are used widely in or by consumers to prevent the occurrence of symptoms following radiotherapy. To evaluate the possible efficacy of health supplements and their potential for use during deep space exploration, a systematic literature review was conducted. A search of PubMed, Cochrane Library and OVID (Medline) databases was conducted, identifying 77 studies that examined therapeutic effects of health supplements on ionizing radiation, of which 15 were eligible for inclusion in the analysis. Health supplements provided no beneficial effects to either functional or biochemical physiological outcomes. However, symptomatic relief was found in a range of irradiated areas of the body from a variety of health supplements following irradiation. The localized actions of both the radiotherapy and the health supplement means further investigations would be required before using these health supplements to protect against wholebody radiation exposure. Further investigations should also focus on the health supplements shown to have the greatest positive effects to address the lack of clinical consensus.
Keywords
Spaceflight; Astronauts; Environmental physiology; Healthcare; RadiotherapyKeywords: Spaceflight; Astronauts; Environmental physiology; Healthcare; Radiotherapy
Introduction
Chronic low dose space radiation poses a major health hazard for astronauts. These risks will be exacerbated in future missions beyond low Earth orbit where the protection of the Earth’s magnetic field is diminished or absent and the longer exposure times will enable radiation damage to accumulate [1]. Space radiation is primarily ionizing radiation, composed of galactic cosmic rays and solar radiation, that consist of free protons, alpha particles and highly energetic, heavy, charged particles (HZE particles) [2]. These particles can alter the structure of atoms and molecules, leaving them charged [3]. In biological tissues, changes to atomic and molecular structures, lead to defects in cellular functions, which leads to the production of reactive oxygen species [4]. The resulting oxidative stress and inflammation damages genetic material, such as breaking the double strands in DNA [5,6]. Incorrect repair of the DNA strands can cause mutation, leading to the production of dysfunctional proteins within the cells, causing cellular dysfunction which in turn impairs tissue function and then organ impairment which poses severe health risks [7]. Relatively little is known about human health following exposure to space radiation due to limited spaceflight beyond low-Earth orbit and the justified ethical issues that would surround Earth-based studies that irradiate humans with whole-body ionizing radiation and technical issues associated with delivery of a space-like radiation in earth.
Although single-species ionizing radiation is a just one component of space radiation, there are rare occasions when humans are exposed to whole-body ionizing radiation (e.g., a nuclear event) [8]. However, one way humans are routinely exposed to ionizing radiation is radiotherapy, which utilizes ionizing radiation to target cancerous cells in a manner that minimally affects non-cancerous areas [9]. Due to the hazardous nature of radiotherapy, it is important to protect the adjacent, non-cancerous tissue [10]. One method used by both clinicians and cancer patients are health/nutritional supplements. While there is a broad range of health supplements it is thought that these are primarily effective as they contain antioxidants which can help repair the oxidative damage caused by ionizing radiation before it causes damage to DNA, tissue and organs [11,12]. Other health supplements appear to offer immunostimulating effects that upregulate immune function to deal with microdamage caused by ionizing radiation or have vascular effects that increase blood flow to affected areas. The exact mechanisms can be hard to establish, as by definition health supplements are unapproved medications containing one or more presumed active ingredients that are yet to be established. Most frequently health supplements are taken orally, rectally or applied topically either prophylactically or after radiation exposure [13]. Health supplements are not regulated and can often vary in dose concentration and amount, leading to their wide availability, with a market value of over USD$25 billion [13-16].
The unregulated and under researched nature of health supplements requires further analysis to determine whether they can be a viable candidate to mitigate the effects of space ionizing radiation, as other, better researched, pharmaceuticals have provided minimal solutions to date. As a result terrestrially based analogues, such as radiotherapy patients, likely provide the best insights for the spaceflight environment. However, health supplements may not necessarily offer the beneficial protection against ionizing radiation they are hypothesized to. For example, a meta-analysis by Chan et al., looking at radiative skin damage in 47 studies that used local and systematic agents to reduce radiation-induced skin reactions found few satisfactory results [17]. Additionally, many current health supplements are used despite limited evidence showing their effectiveness [18,19]. In terrestrial studies, health supplements are used in a variety of manners and dosages in studies of various lengths, with differently symptomatic populations receiving different accumulative radiation doses to treat specific symptoms. However, in spaceflight the unknown factors require being aware of all possible negative effects and understanding how to effectively combat them [13].
Health supplements may provide a safe and effective option to prevent or reduce radiation damage. Therefore, the aim of the current systematic review was to evaluate currently used health supplements that may protect tissues from ionizing radiation that could be implemented in spaceflight. The outcomes of the present study may also have terrestrial applications in radiotherapy as well as occupational and accidental radiation exposure.
Materials and Methods
Search strategy
A systematic search was conducted in PubMed, Embase (OVID) and CENTRAL (Cochrane library) databases in August 2021. The search strategy consists of terms for radiation, pharmaceuticals and randomized controlled trials, combined using Boolean operators (Supplementary information).
Selection criteria
The PICOS (Population, Intervention, Comparison, Outcomes, Study design) framework was used to inform the selection criteria:
• I: Pharmaceutical supplementation prior to during or after partial or whole-body exposure to ionizing radiation [28-37].
• C: No pharmaceutical supplementation with the same exposure to ionizing radiation [38-47].
• O: Functional, biochemical or clinical outcomes to ionizing radiation [48-57].
• S: Randomized controlled trials [58-65].
Study selection and data extraction
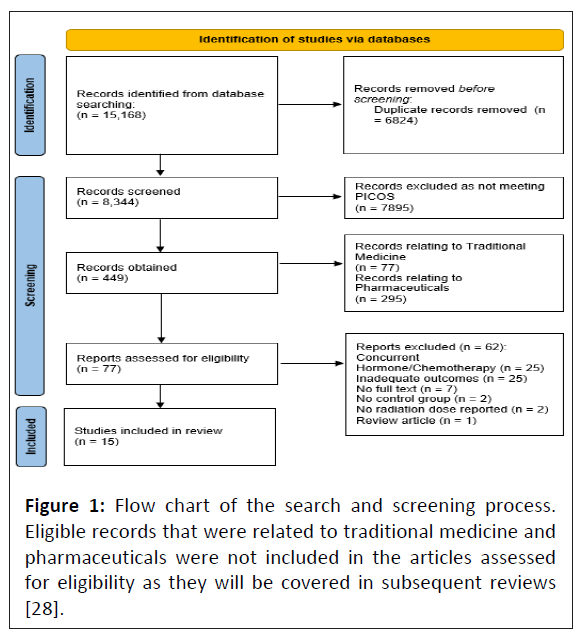
All studies obtained from the search were imported into Rayyan (Web Rayyan QCRI) where they were screened by two reviewers using the inclusion criteria [20]. Initial screening consisted of excluding studies based on the title and abstract. The remaining articles had their full texts assessed for inclusion. In the event of a disagreement between the two reviewers, the discrepancies were resolved by a blinded third reviewer (i.e., each included study was deemed eligible by two reviewers). Studies were excluded if they had participants receiving concurrent cancer-related chemotherapy, immunotherapy or hormone therapy treatment, used ultraviolet radiation, included drugs designed to instigate a negative biological effect (i.e., destroy cancerous tissue) and not matching the PICOS (Figure 1). Due to the number of records after title/abstract screening, the articles were categorized as using health supplements, pharmaceuticals or traditional medicine. Pharmaceuticals and traditional medicine records were put aside to be investigated in subsequent reviews. Health supplements were required to have a theorized active, radioprotective ingredient and were classified by their administration. Articles in which a health supplement was used alongside standard care that was also given to controls was interpreted as the effects of that health supplement.
Data extraction and reporting
Data was extracted from all included studies using a modified version of the Space Biomedicine Systematic Review Methods data extraction and analysis tool (sites.google.com/view/srmethods/ guides) [21]. Extracted data includes study design, health supplement (dose, frequency), population, radiation (dose, frequency) and results. Outcomes were categorized as functional relating to tissue or organ function, biochemical relating to constituents of tissues or organs or symptomatic relating to perceived or measured symptoms.
Assessment of quality of studies
All included studies were randomized controlled trials conducted in adult humans. Each study was evaluated using Cochrane’s risk of bias methodology and scored as high risk, low risk or unclear risk of bias. Uncertainties were discussed between at least two reviewers to achieve a consensus.
Data analysis
Means, standard deviations and sample sizes were extracted from the text and if standard error of the mean or confidence intervals were reported, they were converted into standard deviation as per Cochrane guidelines [22]. Where studies only reported data in figures, a plot reader (WebPlotDigitizer) was used to extract data [23].
Data were analyzed in R for statistics (R 3.6.1) using the meta for package [24]. Due to large divergences between drugs and doses data were assessed for each subgroup. Each outcome measure was assessed using the standardized mean difference to obtain the effect size as Hedges. Effect sizes were categorized in terms of the probability of having a true effect as <0.1 uncertain, 0.1-0.3 small, 0.3-0.5 moderate, 0.5-0.7 large, 0.7-0.9 very large, >0.9 extremely large.
While a meta-analysis was not appropriate given the homogeneity of the research methods, a meta regression analysis was conducted to investigate relationships and heterogeneity statistics were reported, both in accordance with the suggestions of Higgins and Seigfried [25,26].
Random effects meta-regression analyses were run using the restricted estimate of maximum likelihood method to return heterogeneity statistics and the I2 statistics for each subgroup, when at least two studies were being evaluated. Heterogeneity was interpreted using Cochrane guidelines: 0%-40%: Might not be important; 30%-60%: May represent moderate heterogeneity; 50%-90%: May represent substantial heterogeneity; 75%-100%: Considerable heterogeneity [22]. A mixed effects model was also run for each type of outcome with risk of bias and radiation dose as moderators to assess whether they contributed to the size of effects seen.
Forest plots were then generated for each subgroup using a 95% confidence interval, with a random effects model and prediction interval plotted for each subgroup [27]. To standardize effects variables where an increase was negative and variables where a decrease was beneficial, were multiplied by -1 to ensure that a positive effect indicates a beneficial effect in favor of the intervention group and vice versa. As the number of studies with similar designs were low, funnel plots to assess publication bias were deemed unsuitable.
Results
Search results
The initial search returned 15,168 articles, with 6,824 of those being identified as duplicates. Screening of the title and abstract of the remaining 8,344 articles led to the exclusion of 7,895 further articles. The remaining 449 articles were classified according to the type of protective agent used, with 77 classed as health supplements, 295 as pharmaceuticals and 77 as traditional medicine. The pharmaceutical and traditional medicine studies were removed to be analyzed individually in separate reviews. The full text of the 77 health supplement articles were retrieved and screened (Figure 1), resulting in 15 final studies that were appropriate for analysis.
Figure 1: Flow chart of the search and screening process. Eligible records that were related to traditional medicine and pharmaceuticals were not included in the articles assessed for eligibility as they will be covered in subsequent reviews [28].
Characteristics of included studies
Of the included studies 5 reported biochemical outcomes, 11 reported symptomatic outcomes and 1 reported functional outcomes. Studies included had been published between 1994 and 2020. The accumulative mean radiation doses ranged from 35.78 Gy to 69.70 Gy, with 14 studies using fractionated radiation exposure and one study using radioactive iodine (I-131).
Five studies used orally taken health supplements, 4 used a topical gel, 2 used enemas and a drink, ointment, mouthwash and intravenous supplements were each used in 1 study. Each study used a different health supplement apart from three studies that used curcumin, two of which came from the same research group (Table 1). Only two studies reported the timing of the health supplement administration, both of which were topical gels, applied 1-3 h prior to radiation or outside of a 4 h window centered on radiation [29,30]. The small number of studies reporting timing prevented this from being analyzed.
| Application | Health supplement | Dose (0.d-1) | n | Target area | Total dose | Reference |
|---|---|---|---|---|---|---|
| Oral | Curcumin | 3000 mg | 45 | Prostate | 74 Gy | [31] |
| Curcumin | 3000 mg | 85 | Prostate | 74 Gy | [32] | |
| Curcumin | 125 mg | 64 | Prostate | 70 Gy | [33] | |
| Vitamin E | 536 mg | 36 | N/A | 4393 MBq (I-131) | [34] | |
| Lactobacillus rhamnosus | 4500 mg | 205 | Pelvis | 52 Gy | [35] | |
| Topical gel | Boron | 4 x to target area | 50 | Breast | 61 Gy | [30] |
| Curcuma longa | 0.5%, 3 x to target area | 37 | Head/Neck | 54 Gy | [36] | |
| RayGel | Unknown, daily | 32 | Breast | 60 Gy | [29] | |
| Vitamin D | 8000 IU | 45 | Head/Neck | 56 Gy | [37] | |
| Enema | Short-chain fatty acids | 120 mL | 19 | Pelvis | 57.4 Gy | [38] |
| Sodium butyrate | 80 mL | 20 | Pelvis | 35-52 Gy | [39] | |
| Ointment | Aloe vera | 2000 mg | 20 | Pelvis | 52 Gy | [40] |
| Drink | Hydrogen-rich water | 1500-2000 mL | 49 | Liver | 50-65 Gy | [41] |
| Mouthwash | GeneTime® | 4x | 100 | Head/neck | 60 Gy | [42] |
| Intravenous | Glutamine dipeptide | 400 mg/kg BW | 61 | Pelvis | 47 Gy | [43] |
Table 1: Overview of studies included for analysis. All health supplements were taken daily throughout radiotherapy treatment. BW: Bodyweight.
Methodological quality of included studies
All studies were evaluated based on their quality and bias. Most studies did not report blinding of the outcome assessment, therefore in such circumstances, this was removed from the analysis of bias. Three studies were determined to be of high risk, while the rest were low risk (Table 2).
| Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Overall risk of bias | References |
|---|---|---|---|---|---|---|---|
| low | low | low | unclear | low | low | low | [30] |
| low | low | low | low | low | low | low | [37] |
| low | low | low | low | low | low | low | [29] |
| low | low | low | unclear | low | low | low | [34] |
| low | low | low | unclear | low | low | low | [31] |
| low | low | low | unclear | low | low | low | [32] |
| low | low | high | high | low | low | high | [41] |
| low | low | low | low | low | low | low | [36] |
| low | low | low | low | low | low | low | [38] |
| low | low | low | low | low | low | low | [33] |
| low | low | low | low | low | low | low | [40] |
| low | low | low | low | low | low | low | [42] |
| low | low | low | low | low | low | low | [35] |
| low | low | low | low | low | high | high | [39] |
| low | low | low | high | low | low | high | [43] |
Table 2: Risk of bias assessment for the studies included in the study using the Cochrane risk of bias tool.
Main outcome parameters
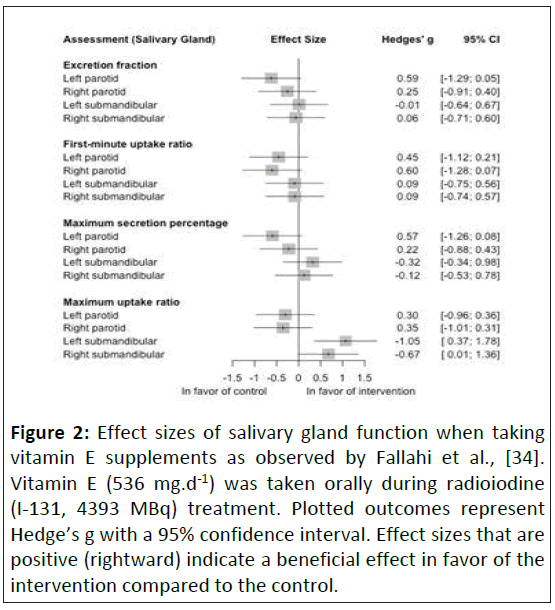
Functional outcomes: Functional changes were assessed in only one study, looking at the effects of vitamin E (536 mg.d-1) on salivary gland function following 4 weeks of I-131 treatment [34]. Effect sizes varied greatly, with no functional outcome showing a clear negative or positive effect of the health supplement, with effect sizes ranging from -0.60 to +1.05. The largest beneficial effect was seen in the maximum uptake ratio of the left submandibular gland (+1.05), but both parotid glands showed slight negative effects (Figure 2).
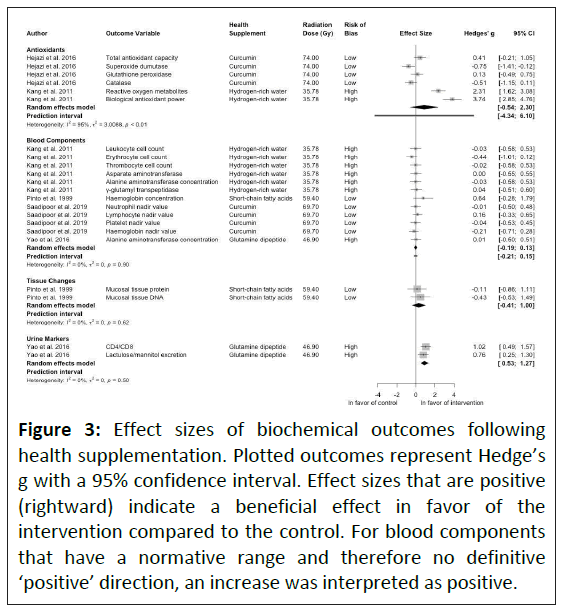
Biochemical outcomes: Biochemical changes were evaluated in five studies, investigating antioxidant behavior, blood components, tissue changes and urine markers. Regarding antioxidants, supplementation using curcumin showed large and very large reductions in catalase (-0.51) and superoxide dismutase (-0.75), respectively, although there was a moderate increase in total antioxidant capacity (+0.41) [32]. Conversely, Kang et al., saw extremely large increases in both reactive oxygen metabolites (+2.31) and biological antioxidant power (+3.74) with the use of hydrogen-rich water, although a relatively low total radiation dose was used [41]. The large variation both between and within studies resulted in the large prediction interval (Figure 2). The hydrogen rich water saw minimal changes in blood components although there was a moderate negative effect on erythrocyte cell count. Similarly small changes were observed using a relatively low dose (120 mg.d-1) of curcumin [33]. A large positive effect on hemoglobin concentration was seen using short-chain fatty acids [38]. Pinto et al., also investigated tissue changes and observed negative effects on both mucosal tissue protein and DNA [38]. Personalized doses of glutamine dipeptide proved effective at improving urine markers of immune health.
Figure 2: Effect sizes of salivary gland function when taking vitamin E supplements as observed by Fallahi et al., [34]. Vitamin E (536 mg.d-1) was taken orally during radioiodine (I-131, 4393 MBq) treatment. Plotted outcomes represent Hedge’s g with a 95% confidence interval. Effect sizes that are positive (rightward) indicate a beneficial effect in favor of the intervention compared to the control.
The meta-regression analysis showed both increased risk of bias (1.43 (0.34-2.51), p=0.010) and elevated radiation dose (0.06 (0.02-0.09), p=0.001) significantly increased the estimated effect sizes. Heterogeneity results suggested considerable heterogeneity (I2=87%), with heterogeneity being much greater in the antioxidant subgroup than in the blood components analysis. Tissue changes and urine markers returned low heterogeneity due to each being composed of a single study.
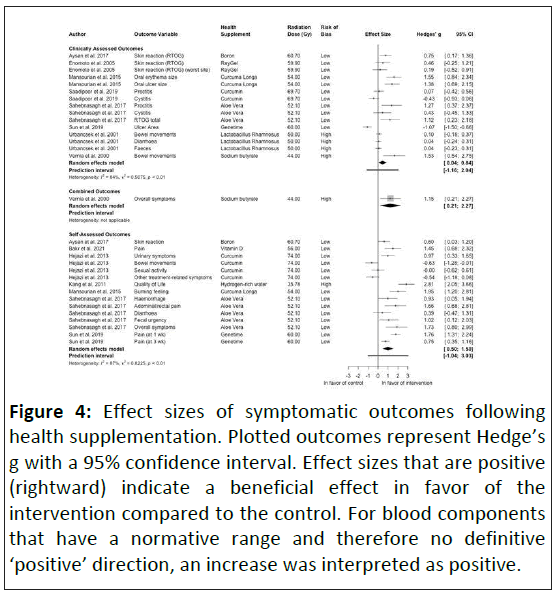
Clinical outcomes: Symptomatic changes varied greatly (range: -1.07 to +2.81) in both clinically assessed and selfassessed outcomes. Skin reaction was improved clinically using topical gels and a rectally applied ointment [29,30,40]. Boron also improved skin reaction when self-assessed albeit at a slightly reduced magnitude, with large and very large effects observed [30]. Proctitis and cystitis were improved following the application of aloe vera (2 g.d-1), but not with curcumin (125 mg.d-1) [33,40]. However using a Curcuma longa gel three times a day led to extremely large effects in reducing oral ulcer and erythema size [36]. Conversely, the use of GeneTime®, a recombinant form of human epidermal growth factor in the form of a mouthwash, induced an extremely large negative effect on ulcer size [42]. GeneTime® reduced patient reported pain, although the size of the effect reduced over time [42]. An extremely large improvement in pain (less pain) was also seen with the topical application of a vitamin D gel twice a day [37]. Self-assessed symptoms in the lower digestive tract were improved by rectally applied aloe vera ointment, but not by curcumin given orally [31,40]. Curcumin supplementation for prostate radiotherapy saw an extremely large improvement in self-reported urinary symptoms, but no effect was seen on reported sexual activity. The largest symptomatic improvement was in quality of life following supplementation with 1.5 L to 2 L of hydrogen-rich water a day (+2.81).
The meta-regression analysis shows that studies with high risk of bias returned higher effect sizes (0.99 (0.20-1.77), p=0.013). Contrary to previous results, higher radiation doses caused a larger positive effect (0.08 (0.05-0.11), p<0.001). The heterogeneity was again high (I2=88%) indicating considerable heterogeneity.
Discussion
Summary of main results
The studies obtained in this systematic review were extremely heterogenous due to the intention of obtaining analogues for spaceflight radiation. As a result of this lack of standardization between studies, radiation varied in both dose and target area with a plethora of health supplements used. No study evaluated whole-body radiation exposure, but rather each study evaluated health supplement efficacy at cancer-specific regions. Of the 57 measures with clear potential for beneficial changes (i.e., not biochemical blood components) 41 showed a beneficial change in favor of the intervention, 2 showed no difference and the remaining 14 showed a beneficial change in favor of the control. Furthermore, 25 showed clear positive effects of the intervention (i.e., confidence interval not crossing 0) and 3 showed clear negative effects. Of these interventions demonstrating clear positive effects 18 were related to symptomatic changes, as were 2 showing clear negative effects. Prediction intervals for each appropriate physiological category (antioxidants, blood components, clinically assessed symptoms and self-assessed symptoms) suggest there is no clear beneficial effect of health supplements.
Quality of evidence
The risk of bias in the assessed studies was predominantly low, likely due to only including randomized controlled trials. Still, 3 trials had a high risk of bias, 1 due to selective reporting of data, 1 due to not blinding the outcome assessment and 1 that did not blind participants or the outcome assessment [39,41,43].
Only one study assessed the ability of functional changes to counter the effects of radiation, focusing on salivary gland function [34]. While large and extremely large effect sizes for maximal uptake ratio were seen in the submandibular glands in patients taking vitamin E supplements, the majority of effects were negative compared to the control group. While the biochemical actions of the antioxidant, vitamin E, would be theorized to provide a radio protective effect, none was seen in salivary gland function at the dose tested [34]. As the radiation dose was localized to the thyroid gland while the vitamin E supplementation was whole-body it is possible that either a higher dose, or a more regionally-targeted supplement could provide a radio protective effect.
While most studies used health supplements with an antioxidant-based mechanism, antioxidant capacity was only measured in two studies. Hydrogen-rich water demonstrated an increase in systemic antioxidant activity, although blinding was not present in this study [41]. Similar findings for hydrogen-rich water have been observed in other domains, notably increasing antioxidant activity to induce beneficial lipid profiles in subjects at risk of metabolic syndrome which were also seen in people with type 2 diabetes or impaired glucose tolerance [44,45]. To a lesser degree curcumin also increased antioxidant capacity with a moderate effect size (Figure 3) [32]. Curcumin is widely used in radiotherapy both for its radioprotective antioxidant ability and its ability to radio-sensitize cancerous cells [46,47]. However, if used in high doses it can increase reactive oxygen species concentrations and therefore is unlikely scalable to larger radiation doses or more widespread radiation [48,49].
The majority of blood components exhibited only minor changes when using a health supplement compared to controls (Figure 3). Of note is the large positive effect size of hemoglobin concentration associated with short-chain fatty acids and the potentially moderate negative effect size of erythrocyte concentration associated with hydrogen-rich water (Figure 3) [38,41]. It is important to note that as blood components sit within normative ranges, the relative direction of the positive and negative change may not necessarily relate to whether the change is beneficial. Short-chain fatty acids are expected to increase hemoglobin concentration as short chain fatty acids induce γ-globin synthesis [50].
Figure 3: Effect sizes of biochemical outcomes following health supplementation. Plotted outcomes represent Hedge’s g with a 95% confidence interval. Effect sizes that are positive (rightward) indicate a beneficial effect in favor of the intervention compared to the control. For blood components that have a normative range and therefore no definitive ‘positive’ direction, an increase was interpreted as positive.
Tissue changes were assessed in only one study, where short chain fatty acids prevented a decline in tissue protein and tissue DNA compared to control [38]. However, in this study the effects of radiation still resulted in reduced tissue protein and DNA, suggesting that short chain fatty acids do not prevent tissue loss, which could be exacerbated by higher radiation doses.
Intestinal permeability was preserved using glutamine dipeptide in a single study, as determined by the lactulose/ mannitol ratio, while immune function, determined by CD4/CD8 ratio, improved [43]. Glutamine provides fuel for lymphocytes, enhancing immune function and protecting the mucosal layer of the intestinal barrier which has been shown to help reduce infection risk and reduce hospital stays in abdominal surgery patients [51-54]. Further studies would be required to confirm these findings however, as the current study that showed this relationship did not blind the outcome assessment.
The largest observed benefits were in symptomatic outcomes, with sodium butyrate, Curcuma longa and aloe vera all inducing extremely large beneficial results in clinically assessed outcomes (Figure 4). Similar results were observed in self-assessed outcomes with vitamin D, hydrogen-rich water, Curcuma longa, aloe vera and GeneTime® (Figure 4). Given the lack of physiological differences this is somewhat surprising, although it should be noted that some of the higher effect sizes seen were in studies with a higher risk of bias. This likely reflects the notion that the radiation is the primary cancer treatment, with health supplements provided to improve quality of life by alleviating symptoms, targeting those that affect patients the most [55-57]. Furthermore, many studies applying health supplements do so as part of curative treatment, as opposed to prophylactic treatment, so it is unclear whether these results could be biased as a result of not being assessed in those with milder symptoms.
Figure 4: Effect sizes of symptomatic outcomes following health supplementation. Plotted outcomes represent Hedge’s g with a 95% confidence interval. Effect sizes that are positive (rightward) indicate a beneficial effect in favor of the intervention compared to the control. For blood components that have a normative range and therefore no definitive ‘positive’ direction, an increase was interpreted as positive.
Relevance to space
Radiation is a major health hazard astronauts will face on missions beyond low Earth orbit [58]. Despite this, there are few strategies to mitigate the effects of radiation damage, with current strategies largely based around reducing operations to preserve health [59]. Current plans of sending humans to Mars (~1000 days) will involve exposures exceeding the cumulative exposure time aboard space stations in low Earth orbit [60]. Not only will the time of exposure be greater in future deep space exploration missions, particularly during sustained habitation missions on the lunar surface, but also the lack of protection from Earth’s magnetic field will leave astronauts exposed to radiation [61]. Physical shielding on Earth is primarily used to avoid radiation exposure in a clinical setting, but the weight (i.e., lead) and relative scarcity of such materials (i.e., water) makes it impractical to use in space and therefore light-weight shielding requires additional strategies to further minimize exposure [62].
The radiation exposures between clinical and extraterrestrial environments differ greatly. The clinical studies analyzed in the current systematic review involved radiation doses of 35-70 Gy at a specific site, whereas in space the radiation dose would be much less (0.48 mGy.d-1), but with the particularity that the whole body would be exposed [65]. Additionally, radiotherapy typically lasts <6 weeks whereas a return Mars mission may involve 500 days of deep space travel, an exposure 12 times greater. While the absorbed dose would still be far less (70 Gy vs. 0.25 Gy, it is unclear what effects this might have as they have never been studied in humans. Therefore, current evidence suggests radiation damage would be less severe. However, on Earth, clinical doses consist of a single type of ionizing radiation, target a specific area and have a controlled and safe, dosage. Conversely, space radiation is composed of many varieties of ionizing radiation, including HZE particles emitted from galactic cosmic rays and solar particle events that have received limited research, particularly on their interaction with biological tissue [66]. In solar particle events the radiation dose can increase to 100 mGy.h-1 or 500 mGy.h-1 if doing extravehicular activity [67]. Solar particle events typically only last hours, but they have the potential to rapidly accelerate the received radiation dose which could result in severe radiation injury throughout the body [68]. While solar particle events can be predicted, the time frame given is relatively short compared to the length of a deep-space mission and the spacecraft would be unable to divert its course to avoid exposure. For this reason, missions will typically be planned in periods of anticipated low solar activity [69]. Regardless of these preventative measures, a high radiation dose resembling that experienced by radiotherapy patients, could feasibly be experienced to the whole body during a mission. In this scenario having onboard medications that reverse the physiological effects and provide symptomatic relief will help to maintain crew health and occupational productivity for the remainder of the mission until they can receive groundbased medical treatment.
A variety of pharmaceutical agents, including health supplements are routinely used on board orbital space stations for a variety of reasons [63,64]. In the current systematic review, there was minimal evidence for the use of health supplements to prevent declines in functional or biochemical markers. Some health supplements offered beneficial responses to symptoms, which could be critical in ensuring operations are uninterrupted from both physical and emotional stress.
However, as space radiation effects the whole body, biological damage, symptoms and consequently their treatment, would not be limited to specific regions as with cancer patients, but instead be more widespread. Therefore, the protective effects of a health supplement when exerted on the whole body may more meaningfully enhance physiological protection or symptomatic relief. Current treatments typically target a specific region, such as glutamine for intestinal damage, gels for skin sites and mouthwashes for dry mouths (Table 1). Ideally a health supplement targeting multiple regions would be used. This is likely to be substances that enter the circulation (i.e. oral supplements, intravenous supplements) rather than topical agents (i.e., gels, ointments) applied to a specific area. The only health supplement evaluated across different radiated regions in this review was curcumin, which acts simultaneously to prevent cancer cell proliferation and preserve non-cancerous cells [70-72]. While numerous studies have shown curcumin to increase survival, reduce tumors and improve quality of life, it remains under investigation as conflicting results exist and results may not be clinically meaningful [73-76]. Whether other health supplements may provide a benefit to other irradiated regions is currently unknown, however health supplements that are distributed systematically are likely the best candidates.
Additionally, in space the health supplements may be metabolized differently in microgravity. If the supplement is metabolized too quickly it can lose its efficacy and if too slowly it can accumulate in the bloodstream, leading to toxicity [12]. Health supplements themselves may be at risk of radiative damage which could destabilize them and reduce their shelf life reducing their effectiveness over time [63]. However, information as to the effectiveness of health supplements in space is limited as of the health supplements flown in space medication information has often been unrecorded and multiple medications are taken (~4.wk-1) which may have effects that interact, thereby masking the true effects of the health supplement [63].
Cumulatively these factors suggest that future research regarding the use of health supplement during spaceflight should look to evaluate the effects of a single health supplement on multiple irradiated areas, especially those that act systematically, as well as whether effects are augmented or suppressed when multiple health supplements are taken at once. Irradiated areas should then be assessed for both areaspecific physiological function that enables tissue health to be tracked, as well as symptomatic improvements relevant to daily quality of life and occupational duties. While using human models of whole-body ionizing radiation to assess these effects is likely unethical, animal models may provide an option to obtain informative insights that can guide best practice. Human trials should look to investigate the timing of health supplement administration in relation to irradiation as this has been underreported but could meaningfully effect the mitigation of radiation damage. Regardless of the interventional population, having a standardized protocol, specific to the radiation anticipated in the spaceflight environment, would help minimize the heterogeneity between studies that was found in this review.
Conclusion
This systematic review suggests limited effects of health supplements on mitigating radiation damage to healthy tissue in regard to functional and biochemical measures. Symptomatic relief may be aided through health supplements, although if these health supplements are shown to only benefit specific areas of the body, additional health supplements may be required to provide whole-body symptomatic relief. The heterogeneity encountered in this systematic review underlies a potential reason for the current clinical lack of consensus and provides further direction for future clinical studies, with appropriate controls, needed to validate the benefits of health supplements shown to have strong positive effects.
Funding
The project was funded by the European Space Agency and KBR GmbH. The funder (KBR GmbH) provided support in the form of salary for the author Tobias Weber.
Competing Interests
The authors declare that there were no competing interests.
References
- Reitz G (2008) Characteristic of the radiation field in low earth orbit and in deep space. J Med Phys 18: 233-243.
[Crossref] [Google Scholar] [Indexed]
- Suparta W, Zulkeple SK (2014) Spatial analysis of galactic cosmic ray particles in low earth orbit/near equator orbit using SPENVIS. J Phys Conf Ser 495: 012040.
- Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM (2014) Effects of ionizing radiation on biological molecules-mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 21: 260-292.
[Crossref] [Google Scholar] [Indexed]
- Ogawa Y, Kobayashi T, Nishioka A, Kariya S, Hamasato S, et al. (2003) Radiation-induced reactive oxygen species formation prior to oxidative DNA damage in human peripheral T cells. Int J Mol Med 11: 149-152.
- Hada M, Georgakilas AG (2008) Formation of clustered DNA damage after high-LET irradiation: A review. J Radiat Res 49: 203-210.
[Crossref] [Google Scholar] [Indexed]
- Terato H, Ide H (2004) Clustered DNA damage induced by heavy ion particles. Biological Sciences in Space 18: 206-215.
[Crossref] [Google Scholar] [Indexed]
- Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461: 1071-1078.
[Crossref] [Google Scholar] [Indexed]
- Mole RH (1984) The LD50 for uniform low LET irradiation of man. Br J Radiol 57: 355-369.
[Crossref] [Google Scholar] [Indexed]
- Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW (2014) Biological response of cancer cells to radiation treatment. Front Mol Biosci 1: 24.
[Crossref] [Google Scholar] [Indexed]
- Prasanna PGS, Stone HB, Wong RS, Capala J, Bernhard EJ, et al. (2012) Normal tissue protection for improving radiotherapy: Where are the Gaps? Transl Cancer Res 1: 35-48.
- Collins N, Tighe AP, Brunton SA, Kris-Etherton PM (2008) Differences between dietary supplement and prescription drug omega-3 fatty acid formulations: A legislative and regulatory perspective. J Am Coll Nutr 27: 659-666.
[Crossref] [Google Scholar] [Indexed]
- Wotring VE (2012) Space pharmacology. Johnson Space Center-Springer: Boston, USA.
- Millman RB, Ross EJ (2003) Steroid and nutritional supplement use in professional athletes. Am J Addict 12: S48-54.
[Crossref] [Google Scholar] [Indexed]
- Anders S, Schroeter C (2017) The impact of nutritional supplement intake on diet behavior and obesity outcomes. PLoS One 12: e0185258.
[Crossref] [Google Scholar] [Indexed]
- Shirazi A, Mihandoost E, Mahdavi SR, Mohseni M (2012) Radio-protective role of antioxidant agents. Oncol Rev 6: e16.
[Crossref] [Google Scholar] [Indexed]
- Yasueda A, Urushima H, Ito T (2016) Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr Cancer Ther 15: 17-39.
[Crossref] [Google Scholar] [Indexed]
- Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, et al. (2014) Prevention and treatment of acute radiation-induced skin reactions: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer 14: 53.
[Crossref] [Google Scholar] [Indexed]
- Williams MS, Burk M, Loprinzi CL, Hill M, Schomberg PJ, et al. (1996) Phase III double-blind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int J Radiat Oncol Biol Phys 36: 345-349.
[Crossref] [Google Scholar] [Indexed]
- Heggie S, Bryant GP, Tripcony L, Keller J, Rose P, et al. (2002) A Phase III study on the efficacy of topical aloe vera gel on irradiated breast tissue. Cancer Nurs 25: 442-451.
[Crossref] [Google Scholar] [Indexed]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan: A web and mobile app for systematic reviews. Syst Rev 5: 210.
[Crossref] [Google Scholar] [Indexed]
- Winnard A, Caplan N, Bruce-Martin C, Swain P, Velho R, et al. (2021) Developing and implementing novel techniques during primary space medicine data systematic reviews. Aerosp Med Hum Perform 92: 681-688.
[Crossref] [Google Scholar] [Indexed]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T (2022) Cochrane handbook for systematic reviews of interventions.
[Google Scholar] [Indexed]
- Rohatgi A (2021) WebPlotDigitizer.
- R Core Team (2020) R: A language and environment for statistical computing. Vienna, Austria.
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557-560.
[Crossref] [Google Scholar] [Indexed]
- Siegfried N, Muller M, Volmink J, Deeks J, Egger M, et al. (2003) Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev: CD003362.
[Crossref] [Google Scholar] [Indexed]
- IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ (2016) Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6: e010247.
[Crossref] [Google Scholar] [Indexed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ: n71.
[Crossref] [Google Scholar] [Indexed]
- Enomoto MT, Johnson T, Peterson N, Homer L, Walts D, et al. (2005) Combination glutathione and anthocyanins as an alternative for skin care during external-beam radiation. Am J Surg 189: 627-630.
[Crossref] [Google Scholar] [Indexed]
- Aysan E, Idiz UO, Elmas L, Saglam EK, Akgun Z, et al. (2017) Effects of boron-based gel on radiation-induced dermatitis in breast cancer: A double-blind, placebo-controlled trial. J Invest Surg 30: 187-192.
[Crossref] [Google Scholar] [Indexed]
- Hejazi J, Taleban FA, Rastmanesh R, Molana H, Ehtejab G (2013) A pilot clinical trial of radioprotective effects of curcumin supplementation in patients with prostate cancer. J Cancer Ther 5.
- Hejazi J, Rastmanesh R, Taleban FA, Molana SH, Hejazi E, et al. (2016) Effect of curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer: A double blinded, randomized, placebo-controlled study. Nutr Cancer 68: 77-85.
[Crossref] [Google Scholar] [Indexed]
- Saadipoor A, Razzaghdoust A, Simforoosh N, Mahdavi A, Bakhshandeh M, et al. (2019) Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytother Res 33: 370-378.
[Crossref] [Google Scholar] [Indexed]
- Fallahi B, Beiki D, Abedi SM, Saghari M, Fard-Esfahani A, et al. (2013) Does vitamin E protect salivary glands from I-131 radiation damage in patients with thyroid cancer? Nucl Med Commun 34: 777-786.
[Crossref] [Google Scholar] [Indexed]
- Urbancsek H, Kazar T, Mezes I, Neumann K (2001) Results of a double-blind, randomized study to evaluate the efficacy and safety of antibiophilus in patients with radiation-induced diarrhoea. Eur J Gastroenterol Hepatol 13: 391-396.
[Crossref] [Google Scholar] [Indexed]
- Mansourian A, Amanlou M, Shirazian Sh, Jahromi ZM, Amirian A (2015) The effect of “Curcuma longa” topical gel on radiation-induced oral mucositis in patients with head and neck cancer. Int J Radiat Res 13: 269-274.
- Bakr IS, Zaki AM, Elâ?ÃÂMoslemany RM, Elsaka RO (2021) Vitamin D oral gel for prevention of radiationâ?ÃÂinduced oral mucositis: A randomized clinical trial. Oral Dis 27: 1197-1204.
[Crossref] [Google Scholar] [Indexed]
- Pinto A, Fidalgo P, Cravo M, Midões J, Chaves P, et al. (1999) Short chain fatty acids are effective in short-term treatment of chronic radiation proctitis: Randomized, double-blind, controlled trial. Dis Colon Rectum 42:788-795.
[Crossref] [Google Scholar] [Indexed]
- Vernia P, Fracasso PL, Casale V, Villotti G, Marcheggiano A, et al. (2000) Topical butyrate for acute radiation proctitis: Randomised, crossover trial. Lancet 356: 1232-1235.
[Crossref] [Google Scholar] [Indexed]
- Sahebnasagh A, Ghasemi A, Akbari J, Alipour A, Lashkardoost H, et al. (2017) Successful treatment of acute radiation proctitis with aloe vera: A preliminary randomized controlled clinical trial. J Altern Complement Med 23: 858-865.
[Crossref] [Google Scholar] [Indexed]
- Kang KM, Kang YN, Choi IB, Gu Y, Kawamura T, et al. (2011) Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res 1: 11.
[Crossref] [Google Scholar] [Indexed]
- Sun H, Zhu X, Li D, Cheng T (2019) Effects of a compound vitamin B mixture in combination with GeneTime® on radiation-induced oral mucositis. J Int Med Res 47: 2126-2134.
[Crossref] [Google Scholar] [Indexed]
- Yao D, Zheng L, Wang J, Guo M, Yin J, et al. (2016) Perioperative alanyl-glutamine-supplemented parenteral nutrition in chronic radiation enteritis patients with surgical intestinal obstruction: A prospective, randomized, controlled study. Nutr Clin Pract 31: 250-256.
[Crossref] [Google Scholar] [Indexed]
- Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N (2010) Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome: An open label pilot study. J Clin Biochem Nutr 46: 140-149.
- Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, et al. (2008) Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res 28: 137-143.
[Crossref] [Google Scholar] [Indexed]
- Okunieff P, Xu J, Hu D, Liu W, Zhang L, et al. (2006) Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys 65: 890-898.
[Crossref] [Google Scholar] [Indexed]
- Javvadi P, Segan AT, Tuttle SW, Koumenis C (2008) The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol 73: 1491-1501.
[Crossref] [Google Scholar] [Indexed]
- Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BVV, Khar A (1999) Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Letters 456: 311-314.
[Crossref] [Google Scholar] [Indexed]
- Galati G, Sabzevari O, Wilson JX, O’Brien PJ (2002) Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 177: 91-104.
[Crossref] [Google Scholar] [Indexed]
- Liakopoulou E, Blau CA, Li Q, Josephson B, Wolf JA, et al. (1995) Stimulation of fetal hemoglobin production by short chain fatty acids. Blood 86: 3227-3235.
[Crossref] [Google Scholar] [Indexed]
- Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, et al. (2010) Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol Res 185: 1037-1044.
[Crossref] [Google Scholar] [Indexed]
- Souba WW, Klimberg VS, Plumley DA, Salloum RM, Flynn TC, et al. (1990) The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res 48: 383-391.
[Crossref] [Google Scholar] [Indexed]
- O’Dwyer ST, Smith RJ, Hwang TL, Wilmore DW (1989) Maintenance of small bowel mucosa with glutamine-enriched parenteral nutrition. J Parenter Enteral Nutr 13: 579-585.
[Crossref] [Google Scholar] [Indexed]
- Zheng YM, Li F, Zhang MM, Wu XT (2006) Glutamine dipeptide for parenteral nutrition in abdominal surgery: A meta-analysis of randomized controlled trials. World J Gastroenterol 12: 7537-7541.
[Crossref] [Google Scholar] [Indexed]
- Pucci C, Martinelli C, Ciofani G (2019) Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 13: 961.
[Crossref] [Google Scholar] [Indexed]
- Henson LA, Edmonds P, Johnston A, Johnson HE, Ng Yin Ling C, et al. (2020) Population-based quality indicators for end-of-life cancer care: A systematic review. JAMA Oncol 6: 142-150.
[Crossref] [Google Scholar] [Indexed]
- Kaiser U, Vehling-Kaiser U, Kück F, Mechie NC, Hoffmann A, et al. (2020) Use of symptom-focused oncological cancer therapies in hospices: A retrospective analysis. BMC Palliat Care 19: 140.
[Crossref] [Google Scholar] [Indexed]
- Patel ZS, Brunstetter TJ, Tarver WJ, Whitmire AM, Zwart SR, et al. (2020) Red risks for a journey to the red planet: The highest priority human health risks for a mission to Mars. NPJ Microgravity 6: 33.
[Crossref] [Google Scholar] [Indexed]
- Chancellor JC, Blue RS, Cengel KA, Auñón-Chancellor SM, Rubins KH, et al. (2018) Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 4: 8.
[Crossref] [Google Scholar] [Indexed]
- Gaffney CJ, Fomina E, Babich D, Kitov V, Uskov K, et al. (2017) The effect of long-term confinement and the efficacy of exercise countermeasures on muscle strength during a simulated mission to Mars: Data from the Mars500 study. J Sports Med 3: 40.
[Crossref] [Google Scholar] [Indexed]
- Chancellor JC, Scott GBI, Sutton JP (2014) Space radiation: The number one risk to astronaut health beyond low earth orbit. Life 4: 491-510.
[Crossref] [Google Scholar] [Indexed]
- Blachowicz T, Ehrmann A (2021) Shielding of cosmic radiation by fibrous materials. Fibers 9: 60.
- Blue RS, Bayuse TM, Daniels VR, Wotring VE, Suresh R, et al. (2019) Supplying a pharmacy for NASA exploration spaceflight: Challenges and current understanding. NPJ Microgravity 5: 14.
[Crossref] [Google Scholar] [Indexed]
- Wotring VE (2015) Medication use by U.S. crewmembers on the international space station. FASEB J 29: 4417-4423.
[Crossref] [Google Scholar] [Indexed]
- Zeitlin C, Hassler DM, Cucinotta FA, Ehresmann B, Wimmer-Schweingruber RF, et al. (2013) Measurements of energetic particle radiation in transit to mars on the mars science laboratory. Science 340: 1080-1084.
[Crossref] [Google Scholar] [Indexed]
- Simonsen LC, Slaba TC, Guida P, Rusek A (2020) NASA’s first ground-based galactic cosmic ray simulator: Enabling a new era in space radiobiology research. PLOS Biol 18: e3000669.
[Crossref] [Google Scholar] [Indexed]
- Onorato G, di Schiavi E, di Cunto F (2020) Understanding the effects of deep space radiation onvnervous system: The role of genetically tractable experimental models. Front Phys 8.
- Hu J, Airapetian VS, Li G, Zank G, Jin M (2022) Extreme energetic particle events by superflare associated CMEs from solar-like stars. Sci Adv 8.
[Crossref] [Google Scholar] [Indexed]
- Jiang J, Wang JX, Jiao QR, Cao JB (2018) Predictability of the solar cycle over one cycle. Astrophys J 863: 159.
- Townsend LW, Wilson JW, Shinn JL, Curtis SB (1992) Human exposure to large solar particle events in space. Adv Space Res 12: 339-348.
[Crossref] [Google Scholar] [Indexed]
- Krigsfeld GS, Sanzari JK, Kennedy AR (2012) The effects of proton radiation on the prothrombin and partial thromboplastin times of irradiated ferrets. Int J Radiat Biol 88: 327-334.
[Crossref] [Google Scholar] [Indexed]
- Giordano A, Tommonaro G (2019) Curcumin and cancer. Nutr 11.
[Crossref] [Google Scholar] [Indexed]
- Mansouri K, Rasoulpoor S, Daneshkhah A, Abolfathi S, Salari N, et al. (2020) Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 20: 791.
[Crossref] [Google Scholar] [Indexed]
- Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, et al. (2006) Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol 4: 1035-1038.
[Crossref] [Google Scholar] [Indexed]
- Greil R, Greil-Ressler S, Weiss L, Schönlieb C, Magnes T, et al. (2018) A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (LipocurcTM) in patients with locally advanced or metastatic cancer. Cancer Chemother Pharmacol 82: 695-706.
[Crossref] [Google Scholar] [Indexed]
- Shafei LKIA, Mohamed IMI, Billa N (2021) Is curcumin at the threshold of therapeutic effectiveness on patients with colon cancer: A systematic review. Front Pharmacol 12: 707231.
[Crossref] [Google Scholar] [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences