The Impact of Saccharomyces boulardii and Kluyveromyces marxianus Postbiotics on Gastrointestinal Health and Psychological Well-Being
Jordi Cune Castellana1, Maria Tintore Gazulla1, Laia Mart�??�?� Melero1, Carlos de Lecea Flores de Lemus1 and Agusti Marti Gil2*
1AB Biotek Human Nutrition and Health, Peterborough, England 2Greenhouse Data Science S.L, Barcelona, Spain
Published Date: 2024-03-11DOI: 10.36648/ipctn.9.1.40
Jordi Cune Castellana1, Maria Tintore Gazulla1, Laia Martí Melero1, Carlos de Lecea Flores de Lemus1 and Agusti Marti Gil2*
1AB Biotek Human Nutrition and Health, Peterborough, England
2Greenhouse Data Science S.L, Barcelona, Spain
- *Corresponding Author:
- Agusti Marti Gil
Greenhouse Data Science S.L, Barcelona,
Spain,
Email: agusti@recerca.com
Received date: February 08, 2024, Manuscript No. IPCTN-24-18643; Editor assigned date: February 12, 2024, PreQC No. IPCTN-24-18643 (PQ); Reviewed date: February 26, 2024, QC No. IPCTN-24-18643; Revised date: March 04, 2024, Manuscript No. IPCTN-24-18643 (R); Published date: March 11, 2024, DOI: 10.36648/ipctn.9.1.40
Citation: Castellana JC, Gazulla MT, Melero LM, de Lemus CF, Gil AM (2024) The Impact of Saccharomyces boulardii and Kluyveromyces marxianus Postbiotics on Gastrointestinal Health and Psychological Well-Being. J Nutraceuticals Food Sci Vol.9 No.1: 40.
Abstract
Background: The gut microbiome plays a pivotal role in overall health, influencing both gastrointestinal and psychological well-being. This study aimed to evaluate the impact of the postbiotics Saccharomyces boulardii ABB S3 and Kluyveromyces marxianus ABB S8 on individuals experiencing gastrointestinal discomfort and to assess their potential in improving gut health and psychological states. The hypothesis was that daily administration of these postbiotics would lead to significant improvements in gastrointestinal symptoms, gut microbiota composition and psychological well-being.
Methods and findings: Seventeen healthy volunteers experiencing gastrointestinal discomfort participated in this one-month study. Participants received a daily capsule of postbiotics. The study assessed changes in gastrointestinal symptoms, gut microbiota composition and psychological well-being through symptomatic ratings, microbiota testing and psychological measures. The intervention led to a significant reduction in gastrointestinal symptom severity with the average digestive distress score decreasing from 4.61 to 1.79. There was also an observed increase in beneficial gut flora abundance and improvements in quality of life and reduced levels of anxiety and somatization. Adverse events were minimal and did not detract from the strong adherence and safety profile of the study. However, the study's limitations include its small sample size and the absence of a control group.
Conclusions: The postbiotic regimen appears to be effective in alleviating gastrointestinal discomfort and improving the composition of the gut microbiota, thereby enhancing participants' psychological well-being and quality of life. These findings suggest the potential of these postbiotics as a therapeutic option for individuals experiencing gastrointestinal distress. Future research with larger sample sizes and controlled study designs is recommended to validate these results and further explore the role of postbiotics in gut health and psychological well-being.
Keywords
Postbiotic; Gastrointestinal well-being; Saccharomyces boulardii ABB S3; Kluyveromyces marxianus ABB sS8; Microbiota; Digestive distress reduction; Gut-brain axis; Psychological well-being; Dietary habits
Introduction
The growing awareness of gut health's crucial role in overall wellbeing has led to a surge in research on the gut microbiota which is known to comprise over 1000 distinct microbial species [1,2].
This has spurred a significant interest in interventions targeted at these microbes to enhance health and prevent diseases. Among these, probiotics, prebiotics, dietary supplements and even fecal transplants are being explored for their healthpromoting potential [3-6]. Probiotics, particularly, have gained wide acceptance for their benefits, including the improvement of gut health and immune support and are available through both prescriptions and over-the-counter options [1,5,7-9].
A notable area of burgeoning interest is the realm of postbiotics, comprising inanimate inactivated microbial cells and their metabolites. Unlike their probiotic counterparts, postbiotics consist of non-living microorganisms but still confer substantial health benefits. Their role is increasingly recognized in ameliorating gastrointestinal disorders, attributed to their immune-modulating, anti-inflammatory, antioxidant and even anti-cancer properties [10-12]. Studies suggest their beneficial effects across various age groups, from infants to adults, highlighting their potential in diverse health contexts [12,13]. For postbiotics to be classified as such they must undergo rigorous evaluation including a comprehensive assessment of their source the inactivation process and the health benefits substantiated by controlled experiments [14-16].
Moreover, recent research indicates that postbiotics may serve as a safe alternative in treating conditions like inflammatory bowel disease and could play a role in preventing diseases like neural disorders, Type 1 diabetes, cancer and various immunological disorders [17,18]. The emerging evidence supports the inclusion of postbiotics as a new frontier in functional food and pharmaceutical research, offering a novel strategy for health improvement and disease prevention [12,19].
In selecting the specific strains of Saccharomyces boulardii ABB S3 and Kluyveromyces marxianus ABB S8 for our study, we focused on their distinct characteristics and the practical advantages they offer for incorporation into functional food products like cereals. These strains were chosen for their proven efficacy in enhancing intestinal well-being, a key factor in the development of health-promoting food items. Saccharomyces boulardii is particularly lauded for its probiotic benefits and its ability to improve gut health and bolster the immune system. It has been specifically designed to withstand various processing conditions, increasing its adaptability for use in a diverse range of products [14,15,20]. Moreover, clinical research has demonstrated that Saccharomyces boulardii effectively aids in the management and prevention of gastrointestinal problems, including antibioticassociated diarrhea and chronic inflammatory bowel diseases, owing to its capability to protect the gut lining and enhance infection resistance [21].
Complementing Saccharomyces boulardii, Kluyveromyces marxianus is recognized for its rapid growth, versatile metabolism and resilience in various food environments, making it a valuable partner in our postbiotic formulation. This particular blend of Saccharomyces boulardii and Kluyveromyces marxianus in a postbiotic form ensures not only the stability and longevity of the product but also maintains its bioactivity, which is essential for its functionality in food products. Their non-living nature makes them particularly valuable in industrial settings, offering a more convenient alternative to probiotics due to their stability and easy storability.
The integration of these strains into foods, especially cereals, provides a convenient way for consumers to include gut health benefits in their daily diet. This approach is in line with the growing consumer trend towards functional foods and the demand for natural, health-promoting ingredients in everyday meals. By selecting these specific strains for their complementary properties and their suitability for food integration, our study contributes to the evolving landscape of functional food development, focusing on natural solutions to enhance gastrointestinal health. Additionally, the nutritional composition of S. boulardii suggests potential prebiotic functions, further enriching its health benefits [5]. The gut microbiota is crucial for nutrient synthesis and absorption and immune system regulation. However, an imbalance in these microorganisms known as dysbiosis, can lead to various health issues. The main focus of our study is to assess how a gastrointestinal postbiotic can alleviate digestive discomfort and modify the gut's microbial landscape. Our method involves an initial evaluation, followed by a 30-days treatment with the postbiotic and a final assessment to determine the intervention's effectiveness [21,22]. This study underscores the intricate relationship between our health and the gut microbiota and the potential to harness this relationship to improve our well-being.
Materials and Methods
The objective of this study was to evaluate the impact of a daily postbiotic treatment on gastrointestinal symptoms, including bloating, nausea and acidity, among others, over a period of 30 days. The secondary objectives were the investigation of effects on the intestinal microbiota and the assessment of the participants' quality of life and psychological wellbeing. Data confidentiality was upheld by utilizing a secure online platform and conducting two in-person visits for each participant. The trial, featured seventeen healthy volunteers who were experiencing gastrointestinal discomfort and agreed to participate.
Eligibility criteria mandated that participants must be at least eighteen years old and exhibit a minimum of two gastrointestinal complaints. Individuals following certain diets such as weight loss ones or using drugs that impact intestinal activity (i.e., antacids, antispasmodics or laxatives) were not considered eligible. Participants had the freedom to withdraw at any point.
Participants were required to supply stool samples before and after the treatment. Between the baseline and final visits, participants responded using their mobile devices to three weekly short surveys assessing safety and adherence to the study. The stool samples were subjected to microbiological investigation. The postbiotic comprising of Saccharomyces boulardii ABB S3 and Kluyveromyces marxianus ABB S8 was given orally in the form of capsules with a dosage of one capsule per day for a duration of one month.
At the beginning of the study groups of five participants were given information obtained their consent and filled out a baseline questionnaire. Weekly monitoring of compliance and its influencing factors was conducted through WhatsApp. Upon the conclusion of the study participants provided a last fecal sample and filled out a concluding questionnaire. The results were compiled for collaborative analysis and subsequently shared with researchers and the sponsoring laboratory.
The study followed ethical standards as outlined by biomedical research regulations, adhering to the principles of good clinical practice, the Helsinki Declaration and the Spanish biomedical research law [23-25]. Participants in the study were comprehensively informed about the nature, purpose, potential risks and benefits of the research. They were given detailed explanations to fully understand the ramifications of their involvement. To ensure informed decision-making, clear, accessible information was provided, allowing participants to ask questions and receive clarifications.
The occurrence of Adverse Events (AEs) and specific situations requiring immediate attention were recorded and promptly reported to the sponsor. This process ensured timely communication of any potential risks or complications arising during the study. The Contract Research Organization (CRO), tasked with overseeing the documentation of adverse events, provided support in maintaining consistent surveillance and record-keeping. The study's monitoring efforts were instrumental in maintaining the integrity of the research, ensuring participant safety and contributing valuable data for the assessment of the treatment's efficacy and safety profile.
In the study, gastrointestinal symptoms were assessed using Visual Analog Scales (VAS), ranging from 0 to 10, where 0 indicated no symptoms and 10 represented the most severe symptoms. To gauge improvements, we compared the scores recorded on day 0 (baseline) with those on day 30, providing a clear, quantifiable measure of symptom changes over the study period. Additionally, both the baseline and day 30 VAS scores for gastrointestinal symptoms were transformed into dichotomous variables for a more rigorous analysis. Scores lower than 1 were classified as 'no symptoms' while scores of 1 or higher were considered indicative of symptom presence. This categorization was designed for analysis using McNemar repeated measures tests, aiming to yield more robust results regarding the changes in symptoms attributable to 30 days of postbiotic intake. This approach allowed for a more detailed understanding of the binary nature of symptom changes, enhancing the interpretability of the treatment's effectiveness.
Additionally, the study utilized the precision microbiome profiling platform, a cutting-edge tool designed for rapid and comprehensive analysis of microbial organisms. This platform enabled us to conduct an in-depth investigation into the diversity and composition of the intestinal microbiome. In a novel approach, ChatGPT-4, developed by OpenAI, was employed to classify intestinal microbes based on their potential health impacts. This AI-driven analysis was instrumental in parsing the complex array of microbiota present in the volunteers' fecal samples. By leveraging ChatGPT-4's capabilities, we could identify and categorize microbes more efficiently, focusing on those with significant health implications. As a result of this comprehensive study, we established a 'beneficial score' for each microorganism, calculated using a multifaceted approach. This score was derived by multiplying the natural logarithm of the relative abundance of each microorganism, as assessed by DNA analysis, by the score generated by ChatGPT. The ChatGPT-derived score for each microorganism ranged from 0 to 9, with 9 indicating the most beneficial effect, 5 being neutral and 0 suggesting potential pathogenicity. We then obtained the global beneficial score by summing the scores of all microorganisms, including those within three specifically identified clusters of interest. Group 1 consisted of butyrate producing species such as Faecalibacterium prausnitzii A2-165, Faecalibacterium prausnitzii M21/2, Roseburia hominis and Ruminococcus bicirculans. Group 2 was composed of Bacteroides species including Bacteroides eggerthii, Bacteroides ovatus, Bacteroides plebeius and Bacteroides uniformis. Group 3 featured Bifidobacterium species specifically Bifidobacterium adolescentis and Bifidobacterium longum. This method took into account various factors such as the presence and relative abundance of beneficial bacteria along with their known or potential effects on human health. Such a scoring system provided a comprehensive and nuanced understanding of how different microorganisms contribute to or detract from the overall health of the gastrointestinal ecosystem.
The assessment of quality of life was conducted using Goldberg’s anxiety and somatization subscales [26-29], specifically targeting a group of young physically fit individuals who volunteered for the study. Dietary patterns were analyzed by comparing study data with Spanish national health survey data [30], transformed to reflect monthly intake, consumption levels and adherence to nutritional guidelines. Descriptive statistics provided a thorough analysis of all variables. Within-group differences were examined using t-tests, Wilcoxon tests and McNemar tests, while between group differences were analyzed with ANOVA (Analysis of Variance) tests, applying a significance threshold of 0.05. In the main inferential statistical analyses, the effect size of the observed changes was calculated. When Student's t-tests for repeated measures were used, Hedges' g correction of Cohen's d was used as the measure of effect size, using the standard deviation of the mean difference as the standardizer. Cohen’s d was used as effect size measure for ANOVA tests. Data analysis was conducted using SPSS v26 and R v4.3.1 software.
Results
Sample
The study sample consisted of 17 participants, predominantly female (10 out of 17). The average age was approximately 45 years with a typical weight of around 72 kg and an average height of 169 cm. Most of the participants had a normal Body Mass Index (BMI), with a smaller fraction falling into the pre-obesity category and a single individual classified as obesity class II. Regarding lifestyle habits, non-smokers formed the majority, while a significant portion engaged in regular physical activity several times a week. Most participants rated their health positively over the past year and the majority reported no chronic health conditions. Average bowel movement frequency was about seven times per week. More detailed demographics and health characteristics of the sample are presented in Table 1.
| n | % | Mean | SD | ||
|---|---|---|---|---|---|
| Sex | Female | 10 | 58.8 | ||
| Male | 7 | 41.2 | |||
| Prefer not to answer | 0 | 0 | |||
| Total | 17 | 100 | |||
| Age | 45.4 | 9.1 | |||
| Weight | 71.6 | 12.6 | |||
| Height | 169.3 | 8.9 | |||
| Body Mass Index (BMI) | 24.9 | 3.8 | |||
| Body Mass Index (BMI)-WHO 8 categories | Severe underweight | 0 | 0 | ||
| Moderate underweight | 0 | 0 | |||
| Mild underweight | 0 | 0 | |||
| Normal weight | 10 | 58.8 | |||
| Pre-obesity | 6 | 35.3 | |||
| Obesity class I | 0 | 0 | |||
| Obesity class II | 1 | 5.9 | |||
| Obesity class III (morbid obesity) | 0 | 0 | |||
| Total | 17 | 100 | |||
| Smoker | No | 10 | 58.8 | ||
| Yes | 4 | 23.5 | |||
| Ex-smoker | 3 | 17.6 | |||
| Total | 17 | 100 | |||
| Frequency with which you do some physical activity in your free time | Does not exercise. Spends free time almost completely sedentarily | 2 | 11.8 | ||
| Occasionally does physical activity or sport | 4 | 23.5 | |||
| Does physical activity several times a month | 1 | 5.9 | |||
| Does sport or physical training several times a week | 10 | 58.8 | |||
| Total | 17 | 100 | |||
| In the last twelve months, would you say your health has been | Very good | 3 | 17.6 | ||
| Good | 11 | 64.7 | |||
| Regular | 3 | 17.6 | |||
| Bad | 0 | 0 | |||
| Very bad | 0 | 0 | |||
| Total | 17 | 100 | |||
| Do you have any chronic or long-term health disease or problem? | None | 13 | 76.5 | ||
| Hypertension | 1 | 5.9 | |||
| Hypothyroidism | 1 | 5.9 | |||
| Thyroid | 2 | 11.8 | |||
| Total | 17 | 100 | |||
| How many times do you have a bowel movement per week | 7.2 | 3.7 | |||
Table 1: Sample characteristics.
Gastrointestinal symptoms
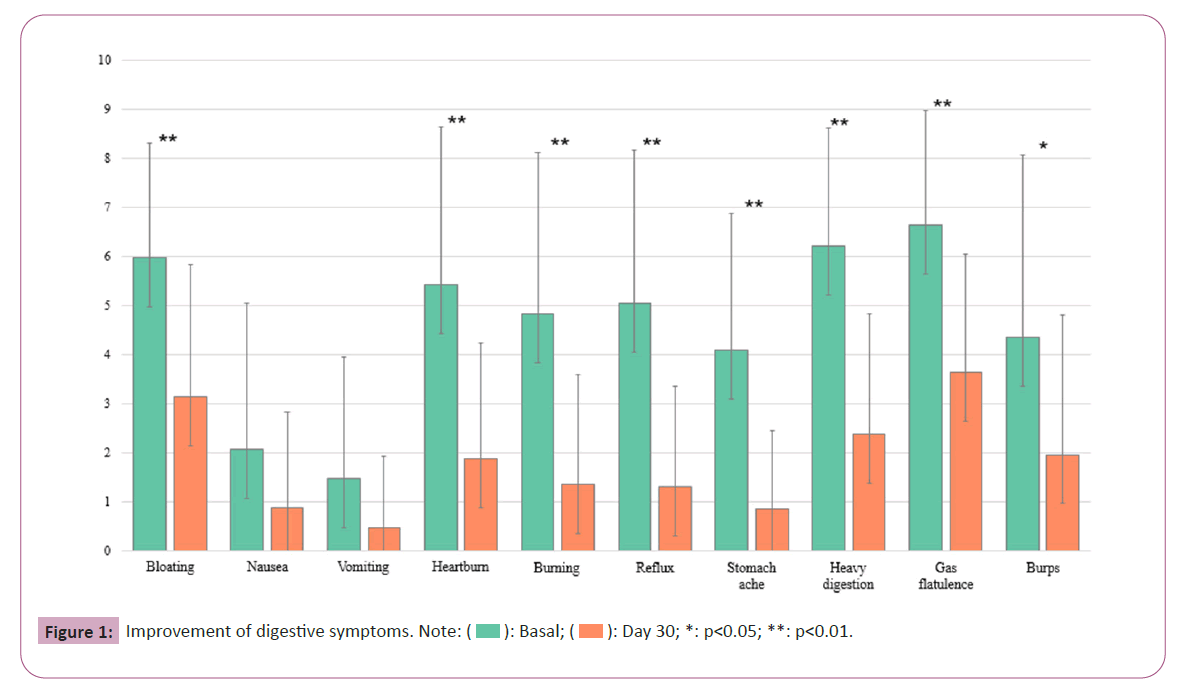
In this research, the impact of a postbiotic regimen on Gastrointestinal (GI) symptoms was quantitatively assessed in a cohort of seventeen individuals over a span of 30 days. The primary endpoint was to determine the efficacy of the treatment through the reduction of GI symptom severity, as quantified by Visual Analog Scale (VAS) scores. The analysis of changes in VAS scores, which signify the participants' subjective symptom severity, revealed significant symptom improvements across most of the recorded categories. This trend was observed as a reduction in mean scores from baseline to day 30, suggesting an overall alleviation of GI discomfort among the participants. The detailed numerical results of these reductions, reflecting mean changes and their statistical significance, are provided in Table 2 and visualized in Figure 1.
| Variable | Baseline | Day 30 | Hedges’ g | 95% CI | p-value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Bloating | 5.98 (2.33) | 3.15 (2.69) | -1.05 | -1.70, -0.48 | <0.001 |
| Nausea | 2.06 (2.98) | 0.87 (1.96) | -0.49 | -0.97, 0.001 | 0.0504 |
| Vomiting | 1.48 (2.46) | 0.46 (1.47) | -0.46 | -0.93, 0.025 | 0.063 |
| Heartburn | 5.42 (3.23) | 1.88 (2.35) | -1.22 | -1.82, -0.59 | <0.001 |
| Burning | 4.84 (3.29) | 1.36 (2.24) | -1.18 | -1.78, -0.56 | <0.001 |
| Reflux | 5.05 (3.13) | 1.31 (2.05) | -1.33 | -1.97, -0.68 | <0.001 |
| Stomach ache | 4.09 (2.8) | 0.84 (1.61) | -1.09 | -1.67, -0.49 | <0.001 |
| Heavy digestion | 6.21 (2.42) | 2.38 (2.46) | -1.28 | -1.90, -0.64 | <0.001 |
| Gas/flatulence | 6.64 (2.34) | 3.65 (2.4) | -1.1 | -1.69, -0.50 | <0.001 |
| Burps | 4.36 (3.72) | 1.96 (2.84) | -0.85 | -1.37, -0.30 | 0.002 |
Table 2: Changes in digestive symptoms.
The McNemar test was applied to assess the cessation of symptoms by day 30, providing additional robustness to the VAS score improvements. A statistically significant decrease in bloating symptoms was noted with the McNemar test yielding a p-value of 0.008, demonstrating a significant resolution in symptoms. For nausea, the McNemar test indicated a positive trend towards symptom resolution with a p-value of 0.05. Vomiting symptoms, while showing a decrease, did not reach statistical significance in the McNemar test, with a p-value of 0.25. The regimen's effectiveness was further evidenced in the treatment of heartburn, where the McNemar test reported a p-value of 0.002, signifying a substantial decrease in symptoms. For burning sensations, the McNemar test supported a significant reduction with a p-value of 0.004.
Reflux symptoms also showed significant improvement, with the McNemar test confirming cessation of symptoms with a p-value of 0.004. Stomach ache and heavy digestion both presented a significant decrease in symptoms, with McNemar test p-values of 0.000 and 0.004 respectively. Although the mean scores for gas/ flatulence significantly decreased, the McNemar test showed a p-value of 0.25, indicating that the cessation of symptoms was not statistically significant at the group level; only 18.75% of cases reported no symptoms by day 30. Burping severity decreased significantly, as evidenced by a t-test result, with a correlation between day 0 and day 30 scores indicating a strong relationship. However, the McNemar test revealed a p-value of 0.063, suggesting no statistically significant change in the frequency of burping symptoms across the study group.
The Wilcoxon signed ranks test was employed to compare the Bristol scale scores between the Basal and Final visits, revealing no statistically significant difference (p=0.334). At the basal visit, the median Bristol scale score was 3.0, with an Interquartile Range (IQR) of 2 to 4. Similarly, at the day 30 visit, the median score remained at 3.0, with an identical IQR of 2 to 4.5 indicating consistency in bowel movement consistency over the course of the study.
Microbiota data
The postbiotic intervention resulted in an increase of the beneficial score from day 0 to day 30 with the mean score rising from 2022.7 to 2476.52. The standard deviation and standard error of the mean decreased, indicating reduced variability among participants. The correlation between the scores on day 0 and day 30 was 0.472, with a two-sided p-value of 0.056. The paired samples test showed a two-sided p-value of 0.029, confirming the statistical significance of the change. Effect sizes were noted to be significant. In Group 1, consisting of butyrateproducing microorganisms (Faecalibacterium, Ruminococcus, Roseburia), a paired t-test revealed an increase in mean levels from 36.73 to 48.71, with a mean paired difference of 11.99. The standard deviation decreased from 20.10 to 12.24. The twosided p-value for the t-test was 0.017. The correlation coefficient between Basal and Final measurements was 0.433 with a onesided p-value of 0.041 and a moderate to large effect.
In Group 2, comprising Bacteroides group microorganisms, there was a significant increase with the basal mean at 26.6 and the day 30 mean at 35.4 resulting in a two-sided p-value of 0.025. The correlation between initial and final levels was not statistically significant with a moderate to large effect having a 95% confidence interval excluding zero. Group 3, consisting of Bifidobacterium sp. microorganisms showed an increase in mean levels from the basal measurement of 29.7 to the final measurement of 37.2 with a two-sided p-value of 0.049. The correlation between the basal and final measurements was 0.690 with a p-value of 0.002. Effect size was g=0.493, supported by 95% confidence intervals that exclude zero. Results of changes in several individual microorganisms from the three studied groups are also presented in Table 3.
| Variable | Baseline | Day 30 | Hedge’ g | 95%CI | p-value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Beneficial score | 2022.7 (883.5) | 2476.5 (367.3) | 0.554 | 0.055, 1.037 | 0.029 |
| G1: Butyrate-producing microorganisms (Faecalibacteria, Ruminococci, Roseburiae) | 36.7 (20.1) | 48.7 (12.2) | 0.618 | 0.110, 1.110 | 0.017 |
| Faecalibacterium prausnitzii A2-165 | 45.2 (31.0) | 57.9 (17.8) | 0.476 | -0.012, 0.951 | 0.056 |
| Faecalibacterium prausnitzii M21/2 | 56.3 (38.3) | 71.72 (21.0) | 0.474 | -0.013, 0.949 | 0.057 |
| Roseburia hominis | 31.5 (23.1) | 40.8 (21.0) | 0.448 | -0.036, 0.920 | 0.07 |
| Ruminococcus bicirculans | 13.8 (23.4) | 24.4 (24.2) | 0.482 | -0.006, 0.958 | 0.053 |
| G2: Bacteroides group micoorganisms (Bacteroides) | 26.6 (15.0) | 35.4 (8.8) | 0.57 | 0.070, 1.056 | 0.025 |
| Bacteroides eggerthii | 3.7 (9.6) | 9.6 (15.8) | 0.465 | -0.022, 0.939 | 0.061 |
| Bacteroides ovatus | 30.3 (20.5) | 40.4 (12.8) | 0.47 | -0.017, 0.945 | 0.059 |
| Bacteroides plebeius | 21.5 (29.6) | 31.5 (29.5) | 0.39 | -0.088, 0.0856 | 0.111 |
| Bacteroides uniformis | 50.8 (24.2) | 59.9 (6.0) | 0.363 | -0.112, 0.827 | 0.136 |
| G3: Bifidobacterium sp. microorganisms (Bifidobacteria) | 29.7 (19.4) | 37.2 (17.1) | 0.493 | 0.003, 0.969 | 0.049 |
| Bifidobacterium adolescentis | 18.8 (25.7) | 26.0 (24.7) | 0.398 | -0.081, 0.865 | 0.104 |
| Bifidobacterium longum | 40.6 (20.8) | 48.4 (16.8) | 0.416 | -0.064, 0.885 | 0.09 |
Table 3: Changes in gut microbiota.
Goldberg subscales and quality of life
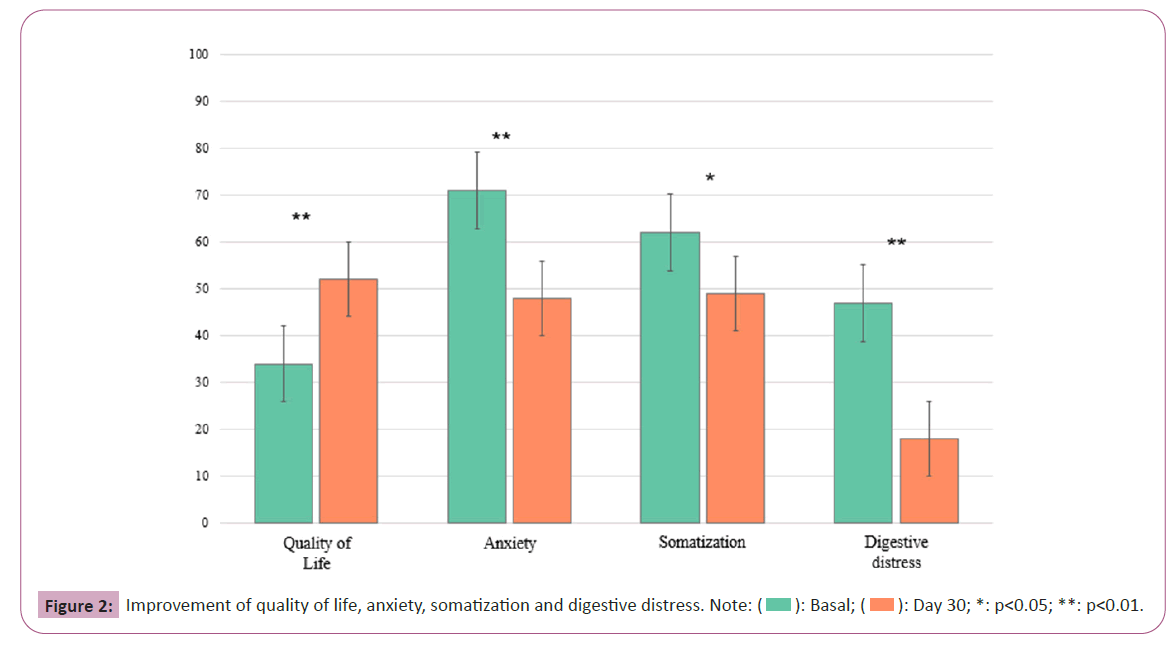
In the study, significant improvements were observed across Goldberg subscales and Quality of Life (QoL) metrics, including somatization and anxiety, as well as reductions in digestive distress over the 30 days period as depicted in Figure 2. Somatization scores showed a notable decrease (p-value=0.008, Hedges’ g=0.7) indicating a significant change with a large effect size. Anxiety levels also decreased (p-value<0.001, Hedges’ g=1.0), suggesting a significant reduction with a very large effect size. Quality of Life (QoL) scores demonstrated a considerable improvement (p-value<0.001, Hedges’ g=1.1), with a very large effect size, implying a meaningful enhancement in participants' life quality. Furthermore, a significant decline in digestive distress was recorded (p-value<0.001, Hedges’ g=1.6), with an extremely high effect size, evidencing the postbiotic regimen's efficacy in managing gastrointestinal symptoms.
The study sample's mean score for excessive non-recommended food consumption was 2.59, higher than the Spanish population's mean of 1.28. ANOVA analysis indicated a significant difference between the study sample and the general Spanish population (p<0.001, Cohen’s d=1.02). Conversely, for deficiency in necessary food, the study sample's mean was 3.41 lower than the Spanish mean of 4.24 with the difference being statistically significant (p=0.016, d=0.582). Regarding protein excess, the study sample's mean score was 1.29, more than double that of the Spanish population, which was 0.63, a difference confirmed as significant by ANOVA (p<0.001, d=0.858). For deficiency of proteins, the study sample's mean was 2.06, lower than the Spanish mean of 3.02, with ANOVA results indicating a significant difference (p<0.001, d=0.961). The mean score for excess of simple sugars was higher in the study group at 1.12, compared to 0.71 for the Spanish population, a statistically significant difference as per ANOVA (p=0.025, d=0.546). However, for carbohydrate deficiencies, both the study group and the Spanish population had similar mean scores (0.71 and 0.74, respectively), with ANOVA showing no significant difference (p=0.8280, d=0.053).
Product questionnaire, safety and volunteer perceived effectiveness
The participants' comment regarding the product was mainly good, with a significant level of approval for the product's format, convenience of dosing and size of the capsules. The compliance rate for daily capsule consumption was 100% with low reports of side effects and 76.5% of individuals did not experience any negative effects. Most of the participants noticed the effect of the postbiotic in the weekly check-ups prior to the day 30 visit. The perceived efficacy of the intervention differed across participants, with 52.9% reporting enhanced digestive well-being while others did not perceive any difference. Certain individuals reported instant effects, whilst others noticed changes either later or not at all. Some individuals also experienced improvements in mood and relief from bloating. To summarize, the study provided a thorough perspective on the positive effects of postbiotics on health markers. The individual replies emphasized the varied influence of the product.
Discussion
The study's primary objective was to assess the efficacy of a postbiotic regimen over 30 days in alleviating gastrointestinal discomforts. The results indicated significant improvements in various GI symptoms, such as bloating, nausea, vomiting, heartburn, burning sensations, reflux, stomachache, heavy digestion, gas/flatulence and burping. The mean scores for these symptoms decreased substantially from baseline to day 30, as shown in Table 2 and Figure 1, suggesting an overall reduction in GI discomfort among the participants. These findings were not only statistically significant but also clinically relevant, evidenced by the effect sizes using Hedges’ g.
Aligning with the contemporary research landscape, our findings resonate with recent studies highlighting the efficacy of postbiotics in managing gastrointestinal disorders. Notably, research by Diez- Gutiérrez et al. and Pirhadi et al. (2021) emphasizes the immune modulating, anti-inflammatory and antioxidant properties of postbiotics [10,11]. These studies, along with ours, suggest that postbiotics, including specific strains like Saccharomyces boulardii and Kluyveromyces marxianus hold substantial promise in treating gastrointestinal discomforts across various demographics.
Specifically, bloating showed a notable reduction, with half of the subjects experiencing alleviation by the study's end. Similarly, there was a decrease in both the intensity and occurrence of nausea. Although vomiting did not exhibit a significant statistical decrease, 75% of individuals initially experiencing this symptom reported cessation by day 30. Heartburn symptoms decreased with a moderate link observed between initial severity and final outcomes, indicating the potential need for tailoring treatment strategies. The study also documented significant reductions in burning, reflux and stomachache symptoms, with most individuals reporting complete recovery or significant improvement. The frequency of burping decreased, with less than half of the participants reporting complete cessation, suggesting an improvement in digestive system functioning. However, this also highlights the need for further research into effective treatments for this symptom. The Bristol scale, used to assess fecal consistency, showed a minor enhancement, albeit not reaching statistical significance.
The results suggest that the postbiotic regimen, including strains Saccharomyces boulardii ABB S3 and Kluyveromyces marxianus ABB S8, may provide a promising therapeutic avenue for individuals experiencing GI discomfort. The significant reductions in symptom severity and observed effect sizes indicate a strong potential for these postbiotics in managing and alleviating GI symptoms. The study also emphasizes the potential of postbiotics as an alternative to probiotics, particularly due to their stability and ease of storage, making them suitable for various applications. Future research may delve into long-term effects, diverse populations and comparisons with other guthealth interventions to further elucidate the role of postbiotics in managing GI symptoms [3,6,21,31].
The study's in-depth microbiota analysis after a 30-day postbiotic intervention revealed transformative changes within the intestinal ecosystem. The significant elevation in the 'beneficial score' is indicative of a positive shift in the gut microbiome towards a more health-promoting state. This score, serving as an indicator of the potential health impact of various microorganisms, displayed decreased variability among participants, suggesting a consistent and uniform response to the postbiotic regimen. Such uniformity in response is a critical observation, as it implies a broadly applicable therapeutic potential of the postbiotic intervention across diverse gut microbiomes.
The clinical implications of the increased presence of butyrateproducing microorganisms, such as Faecalibacterium prausnitzii and Roseburia hominis, are particularly noteworthy. Butyrate, a short-chain fatty acid is pivotal for colon health. It plays a crucial role in maintaining the integrity of the gut barrier, modulating inflammation and may even have protective effects against colon cancer. The augmented levels of these microorganisms could translate into tangible health benefits including improved gut health, reduction in inflammatory states and enhanced overall colon function. Such improvements are vital, considering the increasing prevalence of gastrointestinal disorders and the crucial role of gut health in overall well-being.
The observed increase in Bacteroides group microorganisms also has significant implications. These bacteria are integral to the digestive process, particularly in the metabolism of complex carbohydrates and fibers. Their enhanced presence could potentially lead to a healthier gut environment and improved nutritional absorption. However, given the complex nature of these bacteria, some of which are associated with inflammatory conditions, the clinical impact of these changes must be interpreted with caution. It underscores the intricate balance within the gut microbiome and the nuanced effects that microbial alterations can have on health.
Moreover, the rise in Bifidobacterium species, especially Bifidobacterium adolescentis and Bifidobacterium longum is promising. These bacteria are known for their beneficial impacts, including enhancing gut barrier function, modulating the immune system and producing health-promoting metabolites. Their increased presence might correlate with a comprehensive improvement in gastrointestinal health and broader immune system benefits. This finding aligns with the growing understanding of the gut-immune axis and its implication in overall health and disease prevention.
The aggregate enhancement in the 'beneficial score' and the specific bacterial population changes highlight a crucial shift towards a more beneficial gut environment. This shift is particularly relevant in the context of gut dysbiosis-related conditions suggesting that postbiotics could be a viable intervention for their management or even prevention. The study's findings pave the way for a broader understanding of postbiotics' role in modulating the gut microbiome and their potential therapeutic applications.
Overall, the study reinforces the concept that targeted postbiotic therapy could be a transformative approach to gut health management. By fostering beneficial bacterial groups, postbiotics may offer an efficient strategy to modulate the gut microbiome, a key determinant of overall health. The clinical implications of these microbiota changes highlight the importance of considering the entire gut microbiome's balance and the individual's health status. Future exploration into the long-term effects and interactions of these microorganisms with the host's immune system and other gut residents is essential to fully harness the potential of postbiotics in clinical practice.
In addition to assessing the microbiota, the study examined the effect of the product on the participants' quality of life. Remarkably, there was a substantial increase in the participants' overall well-being, indicating the favorable impact of the intervention. This enhancement encompasses multiple dimensions of life encompassing psychological, bodily and social well-being, hence promoting a more fulfilling and healthier lifestyle for the individuals involved. The study also observed a decrease in somatization, which refers to psychological anguish manifesting as physical symptoms. This suggests a significant reduction in psychosomatic complaints. This decrease can be understood as an enhanced capacity to cope with stress or less psychological distress, resulting in increased overall wellbeing. During the study period anxiety, which is a crucial factor in determining mental health, significantly decreased. The decrease in anxiety has significant consequences, including reduced stress levels, enhanced interpersonal connections and an overall improved standard of living. Finally, the occurrence of intestinal discomfort, which can greatly impair everyday activities, significantly diminished. The robust statistical support for these findings underscores the potential effectiveness of the intervention, indicating opportunities for improving gastrointestinal health and, consequently, quality of life. Overall, the study indicates that the intervention effectively influenced the composition of gut microbiota, resulting in potential advantages that go beyond simply alleviating symptoms and instead lead to significant enhancements in quality of life. These findings present a persuasive argument for conducting additional research on the product's impact on enhancing gut health and general wellness [5,6,32].
The study revealed that participants diverged from conventional Spanish diets, engaging in excessive consumption of nonrecommended foods, proteins and simple carbohydrates. This deviation from a balanced nutritional intake may offer insight into the unusually high prevalence of gastrointestinal discomfort among otherwise healthy and young individuals. The dietary shift towards higher protein and simple carbohydrate intake, coupled with reduced consumption of fiber-rich foods, can disrupt normal gut flora and gastrointestinal processes. This imbalance potentially leads to altered gut motility, increased gas production and a change in the intestinal pH, thereby contributing to the observed gastrointestinal symptoms. Moreover, the overconsumption of processed and non-recommended foods, often high in fats and sugars, can exacerbate inflammation in the gastrointestinal tract, further aggravating these symptoms [2,15,21].
Participants commended the product's use, specifically highlighting its handy packaging and dosage, expressing a preference for gummy alternatives. However, the flavor of the product might need enhancement. Ensuring a high level of aesthetic appeal and ease of use is essential for promoting regular usage and maximizing the health advantages of the product [4,16]. The participants had a high level of adherence, which adds confidence to the outcomes of the study, despite a few individuals experiencing real benefits. The safety profile exhibited exceptional qualities, with just negligible concerns identified, indicating that additional research is needed to explore the product's potential for wider application. The perceived success of the intervention varied, with over half of participants experiencing improvements in their digestive health and a quarter reporting enhanced overall well-being [2,9,20].
Further substantiating our results, the work of Abbasi et al. points to postbiotic components as promising tools for both prevention and treatment strategies in gastrointestinal disorders [12]. Similarly, Tsilingiri et al. suggest that postbiotics may offer a safe alternative for treating inflammatory bowel disease [17]. These studies, coupled with our findings, underscore the potential of postbiotics as a viable, side-effect-minimized therapeutic approach, particularly in conditions like inflammatory bowel disease and other gastrointestinal disorders. The study, while providing valuable insights into the impact of a postbiotic intervention on gastrointestinal and psychological health, has several limitations. Firstly, the small sample size of 17 individuals may not adequately represent the general population. Secondly, the absence of a control group makes it difficult to conclusively attribute improvements to the postbiotic treatment alone. The one-month duration of the study could be insufficient to assess long-term effects or potential side effects. Additionally, the reliance on self-reported measures introduces the possibility of subjective bias. However, this limitation is balanced by the use of objective measurements such as microbiome analysis and validated questionnaires such as the Goldberg questionnaire, that reduce this potential subjective bias. The study's participants, being healthy individuals experiencing gastrointestinal discomfort, also limits the applicability of the findings to a broader or more diverse population. These limitations should be considered in the interpretation of the results and in the design of future studies.
Conclusion
The study's findings present a comprehensive picture of health improvement following a 30-day postbiotic intervention, encompassing gastrointestinal symptom relief, enhanced gut microbiota composition and improved quality of life, including reductions in anxiety and somatization. Initially, participants experienced significant alleviation in a variety of GI symptoms such as bloating, heartburn and nausea, indicating the postbiotic regimen's effectiveness in managing and reducing gastrointestinal discomfort. This symptom relief was paralleled by transformative changes in the gut microbiome, highlighted by a notable increase in the beneficial score.
The shift to a healthier gut environment, marked by increased butyrate-producing and beneficial bacteria, plays a crucial role in enhancing overall gut health and may improve nutrient absorption, immune response and reduce inflammation. Additionally, the study noted significant improvements in participants' quality of life, including reduced anxiety and better stress management. These collective findings underscore the multifaceted impact of postbiotics, not only in alleviating specific gastrointestinal symptoms but also in fostering a balanced gut microbiota and improving psychological health.
References
- Tsilingiri K, Rescigno M (2013) Postbiotics: What else? Wageningen Academic Publishers 4: 101-7.
- Salminen S, Collado MC, Endo A, Hill C, Lebeer S, et al. (2021) The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 18: 649-67.
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A (2019) Mechanisms of action of probiotics. Advances in Nutrition 10: 49-66.
- Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, et al. (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17: 687-701.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, et al. (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11: 506-514.
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, et al. (2017) Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14: 491-502.
- Nataraj BH, Ali SA, Behare PV, Yadav H (2020) Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb Cell Fact 19.
- Moradi M, Molaei R, Guimarães JT (2021) A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzyme Microb Technol 143: 1-10.
- Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W (2020) Postbiotics-a step beyond pre and probiotics. Nutrients 12: 1–17.
- Diez-Gutiérrez L, Vicente LS, Barrón LJ, Villaran M, Chávarri M (2020) Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J Funct Foods 6: 103669.
- Pirhadi M, Alikord M, Mohammadi M, Shariatifar N (2021) Beneficial effects of post-biotics on food products and its effect on human health: A critical review. Plant Biotechnology Persa 3: 56-62.
- Abbasi A, Ghasempour Z, Sabahi S, Kafil H, Hasannezhad P, et al. (2021) The biological activities of postbiotics in gastrointestinal disorders. Crit Rev Food Sci Nutr 62: 5983-6004.
- Morales-Ferré C, Azagra-Boronat I, Massot-Cladera M, Tims S, Knipping K, et al. (2022) Preventive effect of a postbiotic and prebiotic mixture in a rat model of early life rotavirus induced-diarrhea. Nutrients 14: 1163.
- Buts JP, de Keyser N (2006) Effects of saccharomyces boulardii on intestinal mucosa. Dig Dis Sci 51: 1485-92.
- Piqué N, Berlanga M, Miñana-Galbis D (2019) Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int J Mol Sci 20: 2534.
- Oeztuerk H, Schroeder B, Beyerbach M, Breves G (2005) Influence of living and autoclaved yeasts of Saccharomyces boulardii on in vitro ruminal microbial metabolism. J Dairy Sci 88: 2594-600.
- Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, et al. (2012) Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 61: 1007-1015.
- Maguire M, Maguire G (2019) Gut dysbiosis, leaky gut and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics and postbiotics. Reviews in the Neurosciences 30: 179-201.
- Sabahi S, Rad A, Aghebati-Maleki L, Sangtarash N, Ozma M, et al. (2022) Postbiotics as the new frontier in food and pharmaceutical research. Crit Rev Food Sci Nutr: 1-28.
- Czerucka D, Piche T, Rampal P (2007) Review article: Yeast as probiotics-saccharomyces boulardii. Alimentary Pharmacology and Therapeutics 26: 767-78.
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, et al. (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet and diseases. Microorganisms 7: 5-10.
- Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474: 1823-1836.
- (2016) Guideline for good clinical practice E6(R2). International Council for Harmonisation.
- World Medical Association (2013) World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 310: 2191-2194.
- Head of State (2007) Law 14/2007, of July 3, on Biomedical Research. BOE 159: 28826-28848.
- Goldberg DP, Hillier VF (1979) A scaled version of the general health questionnaire. Psychol Med 9: 139.
- Duch CFR, Ruiz de Porras RL, Gimeno RPD, Allué TB, Palou VI (1999) Psychometry of anxiety, depression and alcoholism in primary care. Semergen 25: 209-225.
- Rosenberg E, Lussier MT, Beaudoin C, Kirmayer LJ, Galbaud G (2002) Determinants of the diagnosis of psychological problems by primary care physicians in patients with normal GHQ-28 scores. Gen Hosp Psichiatr 24: 322-327.
- Lobo A, Pérez-Echeverría MJ, Artal J (1986) Validity of the scaled version of the general health questionnaire (GHQ-28) in a Spanish population. Psychol Med 16: 135-140.
- INE (2017) National health survey.
- Generoso SV, Viana ML, Santos RG, Arantes RME, Martins FS, et al. (2011) Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed saccharomyces boulardii. Eur J Nutr 50: 261-269.
- Faria AMC, Weiner HL (2005) Oral tolerance. Immunol Rev 206: 232-59.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
 ): Basal; (
): Basal; (  ): Day 30; *: p<0.05; **: p<0.01.
): Day 30; *: p<0.05; **: p<0.01.