Short-Term Anxiolytic and Pro-Hypnotic Activity of a Tryptic Hydrolysate of Bovine ÃÆà ½Ãâââ¬Ës1-Casein in Patients with Anxiety Spectrum Disorders
Giuseppe Ceraudo, Sonia Cortopassi, Lavinia Rossi, Bernardo Dell’Osso and Liliana Dell’Osso
Giuseppe Ceraudo1*, Sonia Cortopassi1, Lavinia Rossi1, Bernardo Dell’Osso2,3 and Liliana Dell’Osso1
1Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
2Department of Psychiatry, University of Milan; Fondazione IRCCS Ca’ Grande, Ospedale Maggiore Policlinico, Milan, Italy
3Department of Psychiatry and Behavioral Sciences, Bipolar Disorders Clinic, Stanford University, CA, USA
- *Corresponding Author:
- Giuseppe Ceraudo
Clinica Psichiatrica, University
of PisaVia Roma, 57 Pisa, Italy
Tel: +39 3396739062
E-mail: beppeceraudo@yahoo.it
Received Date: June 23, 2017 Accepted Date: August 14, 2017 Published Date: August 30, 2017
Citation: Ceraudo G, Cortopassi S, Rossi L, Dell’Osso B, Dell’Osso L (2017) Short-Term Anxiolytic and Pro-Hypnotic Activity of a Tryptic Hydrolysate of Bovine Αs1-Casein in Patients with Anxiety Spectrum Disorders. J Nutraceuticals Food Sci. Vol. 2 No. 2:7
Abstract
We conducted a prospective open-label study with 100 outpatients who had sought psychiatric consult in private clinical practice for anxiety/sleep in subthreshold/ full blown DSM-IV Anxiety Spectrum Disorders. Clinicians, prescribed for 4 weeks a dietary supplement based on a formulation containing α-casozepine peptide 300 mg/day. The comparison of all rating scales mean scores reported at T0 versus T1 showed a statistically significant decrease (p<0.001). In Clinical Global Impression scale, the 54% of the sample was found to be much improved, 27% minimally improved and 19% showed no change. The 64% of the sample reported an anxiolytic effect, and among the 64 patients with sleep disorders, the 51.5% reported a pro-hypnotic effect. Considering patients in monotherapy with the dietary supplement, an anxiolytic effect was observed in 69.7% while a prohypnotic effect was observed in the 62.5% of the sample. No side-effects were reported during the treatment with no drop-out.
Keywords
α-casozepine; Dietary supplement; No side effects; Novel treatment
Introduction
Anxiety Spectrum Disorders are amongst the most prevalent mental disorders and are responsible for reduced quality of life and significant disability in affected patients [1]. Effective treatment strategies for these conditions include psychotropic compounds (benzodiazepines and pro-serotonergic antidepressants) and psychotherapeutic interventions [2,3]. However, many patients do not have access to or refuse this kind of treatments for different reasons, including pharmacophobia, fear of dependence, economic and other motivations [4-6]. Therefore, novel nutraceutical compounds with potential anxiolytic effects have attracted the interest of researchers over the last years [7,8].
For generations, mothers have given their children a warm glass of milk before going to bed as a way to help them fall asleep. As far back as 1934, this home remedy gained scientific validation when it was observed that people who had taken milk and cornflakes were more likely to enjoy a full night of uninterrupted sleep [9]. Subsequently, Brezinova and Oswald showed, using electroencephalography, that sleep was significantly improved (longer and un-interrupted night sleep-time) in older people, when they had taken a combination of cow milk and cereals before going to bed [10]. In 1997, amongst pediatric researchers, Blass provided further evidence in the field by demonstrating that newborns given an infant formula containing milk fell asleep not solely due to nursing and being held, but specifically to something in milk itself [11]. Just at the beginning of the new millennium, it has been identified what that “something” was. It turned out that nutrients found in cow milk called bioactive peptides, chains of amino acids, exert a sedative effect on the brain and induce sustained sleep patterns [12]. These bioactive milk peptides have been shown to act on the brain GABA-A receptors, which represent the target of one of the most effective classes of sedatives: the benzodiazepines. Specifically, only one peptide named α-casozepine, corresponding to the 91–100 fragment from bovine αs1-casein, expressed affinity for GABA A receptor [13]. In pre-clinical models, milk peptides markedly reduced anxiety and improved sleep in animals subjected to chronic stress [14]. Authors demonstrated that the injection of 3 mg/kg of α-casozepine significantly reduced the epileptic symptoms caused by pentylenetetrazole in rats [15]. An anxiety reduction was also observed when the hydrolysate was tested in the elevated plus-maze and in the conditioned defensive burying rat models. Using selective antagonists of 5-HT1A, D1 and GABA-A receptors, other authors [16] have demonstrated to inhibit α-casozepine anxiolytic effect, suggesting a synergic role of these receptors in the anxiolytic activity observed in mice. The α-casozepine amino acid sequence could be related to the carboxyterminal sequence of the polypeptide diazepam binding inhibitor, an endogenous ligand of the central GABA-A and peripheral-type benzodiazepine receptor, but α-casozepine activity was observed only in central GABA-A receptors [13]. More recently, in a double-blind placebo-controlled trial, α-casozepine showed improvements of stress-related symptoms in a total of 63 female volunteers suffering from at least one potentially stress-related disorder, such as anxiety, sleep problems and general fatigue [17]. After one month, the α-casozepine group significantly reduced their symptoms more than placebo, particularly in digestion, intellectual, cardiovascular, emotional and social problems. As widely known, also sleep disturbances benefit from psychotropic compounds with GABAergic activity. In this perspective, a representative sample of day-time workers from the general population of Japan, reporting insomnia during the preceding six months, was treated with α-casozepine in an observational study [18]. Results showed that α-casozepine improved sleep quality after two weeks of treatment and decreased the sleep latency and the daytime dysfunction after four weeks of treatment.
With the aim to investigate the anxiolytic and pro-hypnotic effect of α-casozepine, we conducted the present open-label prospective short-term study with 100 outpatients affected by Anxiety Spectrum Disorders and treated with α-casozepine for 4 weeks.
Material and Methods
We enrolled 100 outpatients who had sought psychiatric consult in private clinical practice for anxiety symptoms. All patients were screened using the Structured Clinical Interview (SCID-I) [19] to assess the psychiatric diagnosis at recruitment time, considering spectrum condition as a clinical picture which does not necessarily meet all the criteria requested for the diagnosis according to the DSM-IV-TR (APA, 2000) [20]. However, in terms of psychometric assessment, inclusion criteria required a minimum score of 8 at the Hamilton Anxiety Scale [21] and/or of 7 at the Insomnia Severity Index [22]. We considered only patients affected by Anxiety and Mood Spectrum Disorders with subthreshold or fullblown diagnoses in relation to reported anxiety symptoms. Three clinicians, according to patients’ needs, proposed and prescribed a Dietary Supplement (DS) based on a formulation containing α-casozepine peptide at a dosage of 300mg/die (provided by Junia Pharma SRL, Italy). A written informed consent for participation into the study was provided by all patients, and the study protocol was approved by the Ethics Committee of the University of Pisa. With the aim to provide an evaluation over the course of anxious symptoms, we administered to all patients at recruitment time (T0) and after 4 weeks (T1) of treatment with DS the following rating scales: 1) the Hamilton Anxiety Scale (HAM-A), quantifying the severity of anxiety symptoms through the investigation of 14 specific items of the anxiety spectrum; 2) the Insomnia Severity Index (ISI), a rating scale for sleep disorders divided into seven multiple-choice questions on sleep quality and its influences in daily life; 3) the Clinical Global Impression scale (CGI) [23] indicating the overall clinical evaluation by a clinician in terms of severity and improvement, respectively, of the clinical picture as a whole. Finally, 4) the therapeutic evaluation was assessed using the Dosage Record and Treatment Emergent Symptom Scale (DOTES) [24], recording concomitant treatments and the presence of side effects due to the ongoing treatment.
Statistical analysis was performed using SPSS version 21 (USA). Qualitative and quantitative analysis of response were conducted. Considering a clinical response to treatment of anxious symptoms a decrease by half of the HAM-A and/or ISI score at T1 and patients who reported marked improvement to CGI. Response rate was compared according to the diagnosis and in patients who took DS in monotherapy versus add on treatment. Parametric variables were described as mean and standard deviation (SD) and were compared by paired t-test or independent t-test. Categorical variables were compared by χ2 test. Statistical significance was assigned for p<0.05.
Results
Of the 100 outpatients reporting anxiety symptoms (70 females, mean age 39 + 14.7), 53% met a diagnosis of subthreshold Anxious Spectrum Disorder (patients with anxious symptoms not meeting all the criteria necessary for diagnosis of Anxiety and/ or Mood Disorders, according to DSM-IV-TR), 20% had Sleep Disorders, 16% Panic Disorder and 11% were affected by Bipolar Disorders (Table 1).
| Variables | Overall Sample (n=100 ) | % |
|---|---|---|
| Mean age | 39 years (18-78) ds=1.47 | |
| Gender | Females | 70% |
| Occupation | Employed | 60% |
| Student | 20% | |
| Unemployed | 7% | |
| Retired | 13% | |
| Diagnosis | Anxiety Spectrum Disorders | 53% |
| Sleep Disorder | 20% | |
| Panic Disorder | 16% | |
| Bipolar Disorder | 11% | |
| Treatment | Lactium monotherapy | 43% |
| Lactium as add-on treatment | 57% |
Table 1: Demographic and clinical features of the study samples.
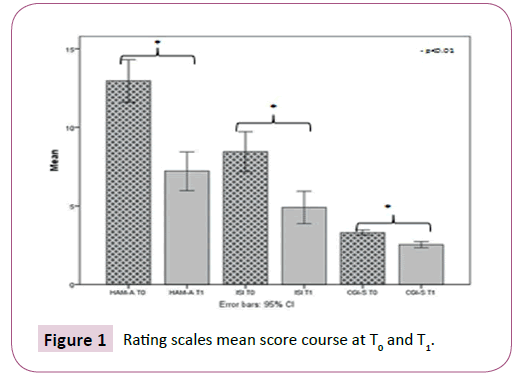
After 4 weeks of treatment, it emerged that 54% of our sample showed a marked improvement at the CGI scale and 27% was found to be minimally improved, while 19% had no change. The comparison of CGI score (T1 versus T0) documented a statistically significant reduction of the mean scores (3.31 ± 0.80 vs 2.54 ± 0.95, p<0.0001). Moreover, we observed a significant reduction of the HAM-A mean scores comparing T1 vs T0 (12.96 ± 6.79 vs 7.21 ± 6.19, p<0.0001). Of the 64 patients with insomnia, the ISI mean score showed a significant decrease from T0 to T1 (8.45 ± 6.48 vs. 4.9h ± 5.19, p<0.0001) (Table 2 and Figure 1).
| 100 patients | T0 | T1 | t | p-value |
|---|---|---|---|---|
| HAM-A | 12.96 ds=6.79 | 7.21 ds=6.19 | 13.8 | <0.0001 |
| ISI | 8.45 ds=6.48 | 4.91 ds=5.19 | 9.44 | <0.0001 |
| CGI | 3.31 ds=0.80 | 2.54 ds=0.95 | 10.86 | <0.0001 |
Table 2: Rating scales mean score course at T0 and T1.
Considering a clinical response to treatment of anxious symptoms a decrease by half of the HAM-A and/or ISI score at T1, we documented a clinical response in the 64% of our sample. When comparing patients taking DS monotherapy (n=43) versus add-on treatment (n=57) (predominantly SSRI or SNRI antidepressants and mood stabilizers), we observed a clinical response in 69.7% of those in monotherapy (HAM-A score) at T1 versus 59.6% who took DS in add-on to other treatments without statistically significant differences.
With regards to the 64 patients with sleep disorders, the 51.5% had a response in terms of ISI score at T1. Patient with sleep disorders and DS in monotherapy represented the 24% of the sample and 62.5% of them showed a clinical response in terms of ISI reduction at T1 while only the 45% of patients who took DS with other treatments, showed a clinical response (p>0.05).
According to the diagnosis, the presence of Panic Disorder was found to be associated with a higher (though not statistically significant) response to treatment than Bipolar Disorder and Anxious Spectrum Disorders respectively (75% vs 72% vs 53%). The analysis of the Dotes scores revealed that DS was not associated with any side effects.
Discussion
In our sample of 100 patients with Anxiety Spectrum Disorders treated with a dietary supplement based on a formulation containing α-casozepine peptide 300 mg/day for 4 weeks, 64% reported an anxiolytic effect, and of the 64 patients with sleep disorders 51,5% reported a pro-hypnotic effect. Considering patients in monotherapy with DS, an anxiolytic effect was observed in 69,7% and 62,5% showed pro-hypnotic effect. The comparison of all rating scales mean scores, at T1 versus T0, showed a statistically significant decrease. Of note, no side-effect was detected in relation to DS treatment. From the analysis of these open-label data, it emerged that DS may be helpful to treat mild/moderate anxiety symptoms. Present findings seem consistent with the results of a recent study reporting sedative and anxiolytic-like effects in pentobarbital-induced sleeping behavior of mice, after the administration of milk collected at night [25].
Serotoninergic antidepressants and benzodiazepines are the most prescribed pharmacological treatment in anxiety disorders [26-28]. Even though in appearance they have two distinct mechanisms of action, serotoninergic antidepressants inhibit glutamatergic neurons of the amygdala through increased extracellular serotonin levels [29]. This inhibition engenders anti-anxiety action. Benzodiazepines also inhibit the amygdala facilitating GABAergic activity, thereby reducing fear and anxiety. The inhibitory action on the amygdala might be, therefore, a common mechanism of anti-anxiety treatments [29]. However, both treatments induce different side effects, serotonergic antidepressants being often responsible for sexual dysfunctions, nausea and weight gain [30], whereas benzodiazepines for tolerance/dependence, somnolence and cognitive deficits [31]. With the aim to assess the evidence about the efficacy of antidepressants and benzodiazepines in adults with Panic Disorder, a recent review analyzed 35 double-blind randomized controlled trials, including 6785 participants overall [32]. Authors found low-quality evidence suggesting no difference between antidepressants and benzodiazepines in terms of response rate. In addition, very low-quality evidence suggested a benefit for benzodiazepines compared to antidepressants in terms of dropouts due to any cause, even if confidence interval ranges from almost no difference to benefit with benzodiazepines. From this perspective, it clearly emerges the need to develop additional treatments with proven efficacy and tolerability in anxiety disorders like Panic Disorder. Even psychotherapeutic interventions are not always easily accessible for patients with anxiety disorders for economic motivations or in light of lack of trust in the therapist or to the effectiveness of treatment [33].
Nutraceutical compounds represent a novel area in a new era of therapeutics for Anxiety and Mood Disorders, in which natural compounds, generally safe and well tolerated, may become a valid treatment option in monotherapy or in add-on to benzodiazepines/antidepressants [34]. Patients frightened by psychopharmacological treatments, patients reporting side-effects beyond minimal dosages, subjects with poor adherence or with unsatisfactory response to treatment can benefit from natural compounds. In particular, those which contains α-casozepine might be used in the acute treatment phase of Anxiety Disorders in add-on to antidepressants and/ or benzodiazepines as well as in monotherapy in prodromal or in maintenance phases and to treat residual symptoms. The potential advantages, which need to be assessed under controlled conditions, may be represented by a greater containment of side effects for lower dosages and therefore a greater adherence to ongoing treatment, from a reduction of the duration of overall treatment and a greater ease to their suspension. In addition, the possibility of being able to prescribe DS to special populations like elderly, child/adolescents, pregnant women and patient with conditions of poor health might be worthy of specific evaluation.
Furthermore, from this study it emerged a double effect, anxiolytic and pro-hypnotic, attributed to DS that misses to antidepressants and that benzodiazepines tend to lose overtime. A dietary supplement based on a formulation containing α-casozepine could be a valid option in the treatment of anxiety spectrum disorders in monotherapy or in add-on to serotoninergic antidepressants and/or benzodiazepines.
In the interpretation of the presented results, the following methodological limitations need to be taken into account. The study was not conceived as a controlled randomized study and it was specifically focused on the short-term treatment. In addition, the studied population was not homogenous in terms of diagnosis, severity, comorbidities and concomitant treatment, being composed by diagnostic subgroups that in some cases were too small in order to detect a statistically significant difference.Nonetheless, we believe that the present uncontrolled report may pave the way for future controlled investigation of the anxiolytic and pro-hypnotic effects of DS in patients with Anxiety Disorders.
Conclusion
The present open-label study found a significant short-term anxiolytic and pro-hypnotic effect of DS in patients with Anxiety Spectrum Disorders, and in subjects with Panic Disorder and Sleep Disorders. The absence of side effects of this natural compound can improve the compliance to treatment, and might be worthy of investigation amongst special populations like elderly, child-adolescents and pregnant women. Future doubleblind, randomized controlled studies are needed to evaluate the efficacy and safety of DS treatment in the long-term and in different populations.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors report no conflicts of interest in relation to the content of the present study.
References
- Baldwin DS, Allgulander C, Altamura AC, Angst J, Bandelow B, et al. (2010) Manifesto for a European anxiety disorders research network. Eur Neuropsychopharmacol 20: 426-432.
- Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper P, et al. (2012) WFSBP Task Force on Mental Disorders in Primary Care and WFSBP Task Force on Anxiety Disorders, OCD and PTSD. Guidelines for the pharmacological treatment of anxiety disorders, obsessive–Compulsive disorder and post-traumatic stress disorder in primary care. Int J Psychiatry Clin Pract 16: 77-84.
- Clark DM (2011) Implementing NICE guidelines for the psychological treatment of depression and anxiety disorders: The IAPT experience. Int Rev of Psychiatry 23: 318-327.
- Schallemberger JB, Colet CF (2016) Assessment of dependence and anxiety among benzodiazepine users in a provincial municipality in Rio Grande do Sul, Brazil. Trends Psychiatry Psychother 38: 2.
- Degli Esposti L, Piccinni C, Sangiorgi D, Fagiolini A, Buda S (2015) Patterns of antidepressant use in Italy: Therapy duration, adherence and switching. Clin Drug Investig 35: 735-742.
- Kostev K, Rex J, Eith T, Heilmaier C (2014) Which adverse effects influence the dropout rate in selective serotonin reuptake inhibitor (SSRI) treatment? Results for 50.824 patients. Ger Med Sci 16: 15.
- Nabavi SM, Daglia M, Braidy N, Nabavi SF (2015) Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr Neurosci 1:2.
- Slyepchenko A, Carvalho AF, Cha DS, Kasper S, Mc Intyre RS (2014) Gut emotions - Mechanisms of action of probiotics as novel therapeutic targets for depression and anxiety disorders. CNS Neurol Disord Drug Targets 13: 1770-1786.
- Laird DA, Drexel H (1934) Experimenting with food and sleep: Effects of varying types of foods in offsetting sleep disturbances caused by hunger pangs and gastric distress-children and adults. J Am Diet Assoc 10: 89-94.
- Brezinova V, Oswald I (1972) Sleep after a bedtime beverage. Br Med J 202: 431-433.
- Blass EM (1997) Infant formula quiets crying human new-borns. J Dev Behav Pediatr 18: 162–165.
- Clare DA, Swaisgood HE (2000) Bioactive milk peptides: A prospectus. J Dairy Sci 83: 1187-1195.
- Miclo L, Perrin E, Driou A, Papadopoulos V, Boujrad N, et al. (2001) Characterization of alpha-casozepine, a tryptic peptide from bovine alpha(s1)-casein with benzodiazepine-like activity. FASEB J 15: 1780-1782.
- Guesdon B, Messaoudi M, Lefranc-Millot C, Fromentin G, Tome D, et al. (2006) A tryptic hydrolysate from bovine milk alpha S1-casein improves sleep in rats subjected to chronic mild stress. Peptides 27: 1476-1482.
- Violle N, Messaoudi M, Lefranc-Millot C, Desor D, Nejdi A, et al. (2006) Ethological comparison of the effects of a bovine alpha s1-casein tryptichydrolysate and diazepam on the behaviour of rats in two models of anxiety. Pharmacol Biochem Behav 84: 517-523.
- Mizushige T, Sawashi Y, Yamada A, Kanamoto R, Ohinata K (2013) Characterization of Tyr-Leu-Gly, a novel anxiolytic-like peptide released from bovine αS-casein. FASEB J 27: 2911-2917.
- Kim JH, Desor D, Kim YT, Yoon WJ, Kim KS, et al. (2007) Efficacy of alphas1-casein hydrolysate on stress-related symptoms in women European Journal of Clinical Nutrition; 61: 536–541.
- De Saint-Hilaire Z, Messaoudi M, Desor D, Kobayashi T (2009) Effects of a bovine alpha S1-casein tryptic hydrosylate (CTH) on sleep disorder in Japanese general population. The Open Sleep Journal 2: 26-32.
- First MB, Spitzer R, Gibbon M, Williams JW (2002) Structured clinical interview for DSM-IV-TR Axis I disorders, Research version, Patient Edition. (SCID-I/P) In: New York Biometrics Research, New York State Psychiatric Institute, USA.
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders (4th edn), Text rev.).
- Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55.
- Bastien CH, Vallières A, Morin CM (2001) Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2: 297-307.
- Guy W (1976) ECDEU Assessment Manual for Psychopharmacology Revised (DHEW Publ No ADM 76–338) pp. 218–222.
- Garvey CA, Gross D, Freeman L (1991) Assessing psychotropic medication side effects among children. A reliability study. J Child Adolesc Psychiatr Ment Health Nurs 4: 127-131.
- De la Peña IJ, Hong E, De La Peña JB, Kim HJ, Botanas CJ, et al. (2015) Milk collected at night induces sedative and anxiolytic-like effects and augments pentobarbital-induced sleeping behavior in mice. J Med Food 18: 1255-1261.
- Reinhold JA, Rickels K (2015) Pharmacological treatment for generalized anxiety disorder in adults: An update. Expert Opin Pharmacother 16: 1669-1681.
- Locke AB, Kirst N, Shultz CG (2015) Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician 91: 617-624.
- Cloos JM, Ferreira V (2009) Current use of benzodiazepines in anxiety disorders. Curr Opin Psychiatry 22: 90-95.
- Inoue T (2012) Pharmacotherapy of anxiety disorders. Seishin Shinkeigaku Zasshi 114: 1085-1092.
- David DJ, Gourion D (2016) Anti-depressant and tolerance: Determinants and management of major side effects. Encephale 13: 30126-30129.
- Murphy Y, Wilson E, Goldner EM, Fischer B (2016) Benzodiazepine use, misuse, and harm at the population level in Canada: A comprehensive narrative review of data and developments since 1995. Clin Drug Investig 36: 519-530.
- Bighelli I, Trespidi C, Castellazzi M, Cipriani A, Furukawa TA, et al. (2016) Antidepressants and benzodiazepines for panic disorder in adults. Cochrane Database Syst Rev 12: 9.
- Berk M, Parker G (2009) The elephant on the couch: side-effects of psychotherapy. Aust NZ J Psychiatry 43: 787-794.
- McCabe D, Colbeck M (2015) The effectiveness of essential fatty acid, B vitamin, Vitamin C, magnesium and zinc supplementation for managing stress in women: A systematic review protocol. JBI Database System Rev Implement Rep 13: 104-118.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences