Proprietary Delivery System of a Novel Supplement Reduced Vasomotor Symptoms in Menopausal Women
Todd Dorfman, Amber Krogsrud, Alison Gracom, Russell Glenn, Justin Hai
Cedalion Health, Boulder, Colorado, United States of America Peptide RX, Palm Beach Gardens, FL, United States of America Complete Care Medical Group, Inc., Irvine, CA, United States of America Rebalance Health Inc., Boulder, Colorado, United States of America
Published Date: 2024-02-16DOI:10.36648/ipctn.9.1.38
Todd Dorfman1, Amber Krogsrud2, Alison Gracom3, Russell Glenn4*, Justin Hai4
1Cedalion Health, Boulder, Colorado, United States of America
2Peptide RX, Palm Beach Gardens, FL, United States of America
3Complete Care Medical Group, Inc., Irvine, CA, United States of America
4Rebalance Health Inc., Boulder, Colorado, United States of America
- *Corresponding Author:
- Russell Glenn
Rebalance Health Inc., Boulder, Colorado,
United States of America
E-mail: rglenn@rebalancehealth.com
Received date: January 16, 2024, Manuscript No. IPCTN-24-18552; Editor assigned date: January 19, 2024, PreQC No. IPCTN-24-18552 (PQ); Reviewed date: February 02, 2024, QC No. IPCTN-24-18552; Revised date: February 09, 2024, Manuscript No. IPCTN-24-18552 (R); Published date: February 16, 2024, DOI: 10.36648/ipctn.9.1.38
Citation: Dorfman T, Krogsrud A, Gracom A, Glenn R , Hai J (2024) Proprietary Delivery System of a Novel Supplement Reduced Vasomotor Symptoms in Menopausal Women. J Nutraceuticals Food Sci Vol.9 No.1: 38.
Abstract
Objective: To evaluate a supplement system for the reduction of vasomotor symptoms in menopausal women.
Methods: A prospective, open-label, IRB-approved study was conducted over 12 weeks to evaluate the efficacy of a supplement system in reducing hot flashes. Females with a mean age of 53, ranging from 41 to 58, who had not used hormonal therapy in the past 12 months and were currently experiencing hot flashes, were included in the study. Participants were given a 90-day supply of the supplement system, consisting of three lozenges per day, reported on the number of hot flashes, and provided feedback on a daily questionnaire. Efficacy was determined by the mean daily change from baseline in frequency of vasomotor symptoms. Adverse events were collected at every visit.
Results: Data analysis showed a significant decrease in the mean number of hot flashes from baseline. At baseline, the mean number of hot flashes was 8.70 and the mean change in the number of hot flashes at weeks 1, 2, 4, 8, and 12, respectively, were -3.97, -4.68, -5.45, -6.31 and -6.94. A majority of participants stated improved disposition and mood, more consistent sleep, a feeling of restfulness upon waking, reduced anxiety with better stress response, and enhanced focus and alertness with reduced brain fog. Additionally, 97% of participants stated they would recommend this supplement system to others looking for an effective solution to improve their menopausal symptoms.
Conclusion: A supplement system using a proprietary Directline® delivery method via a lozenge demonstrated a significant decrease in vasomotor symptoms over a 12-week study. Additionally, women reported improvements in their mood, disposition, sleep, stress response and cognitive function. This supplement system is a viable alternative to hormonal or non-hormonal therapies for mitigating vasomotor symptoms in menopausal women.
Keywords
Menopause; Hot flashes; Vasomotor symptoms; Sleep
Introduction
Menopause impacts millions of women annually and the most prevalent symptoms experienced are hot flashes [1,2]. Various delivery systems, such as injections, creams, pills, and capsules, are utilized to administer therapies and supplements; however, the most prevalent is the use of pills or capsules. The absorption of supplements from pills or capsules is difficult unless doses are high and delivery mechanisms contain protective additives. A recent case study published in the Journal of Vitamins and Minerals showed that the proprietary Directline® delivery method utilized in the Rebalance Health formulations produced a more substantial and viable alternative for the delivery of necessary ingredients, allowing for maximum absorption and significantly higher bioavailable outcomes via lozenge [3]. Utilizing the Directline® delivery technology, a subsequent case study was completed using a supplement system for menopausal women. This case study demonstrated a significant reduction in menopausal symptoms, including hot flashes, insomnia and irritability. Additionally, an improvement in day-to-day physical and mental function was reported. Blood serum estradiol levels increased by over 360% in 2 months compared to baseline. These case study findings suggested that incorporating the Directline® delivery, in conjunction with the supplement system, may effectively manage menopausal symptoms in women. Therefore, a more extensive study was conducted to determine if the supplement system could reduce the incidence of vasomotor symptoms in menopausal women.
Materials and Methods
This prospective, open-label study was conducted over 9 months from February 2023 to October 2023 and was approved by an independent institutional review board prior to initiation. Eligible participants were menopausal women experiencing vasomotor symptoms who had not been on hormone therapy or peptide therapy in the past 12 months and did not have a history of low cortisol levels, autoimmune disease, or abnormalities in the buccal mucosa. Women who had used anti-fungal products within the 30 days or any investigational drug or device within the 60 days before the first dose of the study product were excluded. Pregnant, breastfeeding or women planning on becoming pregnant were also excluded from the study.
Interested females reviewed and completed the informed consent and the medical history screening form. Participants who met the criteria for enrollment were dispensed the supplement system, which consisted of three lozenges per day over 12 weeks. Participants were instructed to take 1 lozenge in the morning, 1 in the afternoon and 1 in the evening. During this study, dose increases were permitted according to the participants’ assessment and the investigators' discretion.
Assessments
Participants completed a daily questionnaire detailing the number of hot flashes experienced, adverse reactions and any additional feedback. At the end of the study, participants completed a supplemental questionnaire to assess changes in disposition/mood, sleep, focus, energy, stress/anxiety, changes in indigestion, libido and skin appearance.
Data analyses
Statistical analyses were performed by an independent consultant using Graph Pad Prism Statistical Software (Graph Pad Software, LLC, CA, USA). Analyses included descriptive statistics and one-way ANOVA (Analysis of Variance) to determine significant differences in the number of hot flashes experienced throughout the study.
Results
104 participants were enrolled in the study, and 5 participants withdrew consent within the first 2 weeks of the study due to personal circumstances; therefore, 99 were included in the data analysis. The mean age of the women in the study was 53, with ages ranging from 41 to 58.
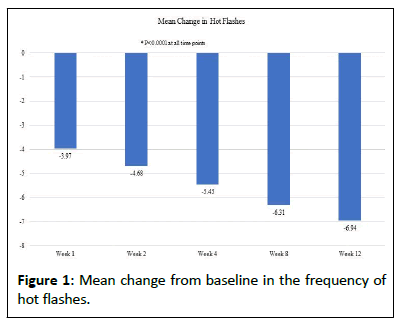
During the 12-week study, the analysis indicated a significant decrease in the mean number of hot flashes from baseline. At baseline, the mean number of hot flashes was 8.70, and the mean change in the number of hot flashes at weeks 1, 2, 4, 8, and 12, respectively, were -3.97, -4.68, -5.45, -6.31, and -6.94 (Figure 1).
Throughout the analysis, it was observed that the mean change in the average daily number of hot flashes experienced before initiating the supplement regimen, as compared to the follow-up weeks, demonstrated a statistically significant decrease.
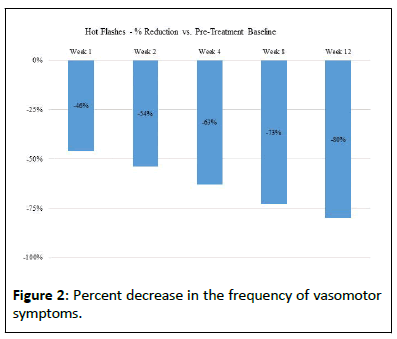
This data analysis equates to a 46% reduction in hot flashes at week 1, 54% at week 2, 63% at week 4, 73% at week 8, and 80% at week 12 (Figure 2).
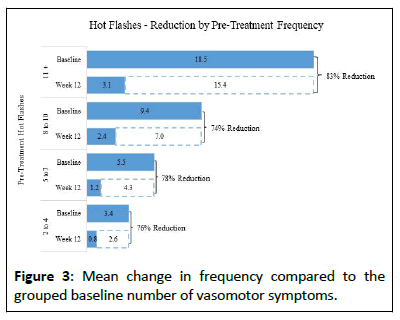
The data was further analyzed by the baseline average number of daily hot flashes. The data was divided into 4 groups based on the number of daily hot flashes reported at baseline. Group 1 n=25 (2-4), Group 2 n=26 (5-7), Group 3 n=28 (8-10) and Group 4 n=20 (11+). All groups had a statistically significant reduction in hot flashes from week 1 through week 12, the mean change in hot flashes at weeks 1, 2, 4, 8, and 12 was as follows: Group 1 (2-4): -1.12, -1.88, -2.08, -2.21, -2.65; Group 2 (5-7): -1.88, -2.65, -3.27, -4.19, -4.35; Group 3 (8-10): -4.25, -4.89, -5.14, -6.26, -7.00; Group 4 (11+): -9.90, -10.54, -12.96, -14.26, -15.38 (Figure 3).
Questionnaire
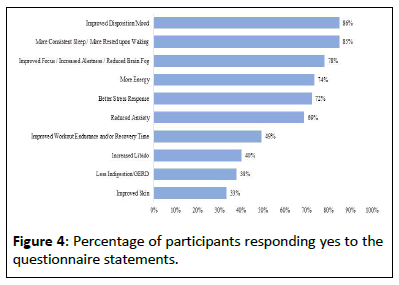
Participants completed a final questionnaire assessing for improvements in additional symptoms, if present, before starting the study. Most participants stated improved disposition and mood, more consistent sleep, a feeling of restfulness upon waking, reduced anxiety with better stress response and enhanced focus and alertness with reduced brain fog. Additionally, 97% of participants stated they would recommend this supplement system to others looking for an effective solution to improve their menopausal symptoms (Figure 4).
Adverse events
During the study, no significant adverse reactions were reported. Two participants experienced mild headaches and mild nausea. Both issues were resolved by reducing the dose to half and then slowly increasing it back to the full dose or by reiterating the instructions for taking the supplements with a meal.
Dose adjustments
34 participants had dose increases throughout the study due to low efficacy with 1 lozenge. 16 participants increased the morning and afternoon doses, 11 increased the nighttime amount and 8 increased the morning, afternoon and nighttime doses (Tables 1a-1c)
| Participant no | Increased from 1 to 2 lozenges (morning and afternoon doses) |
|---|---|
| 5 | Day 45 |
| 9 | Day 81 |
| 10 | Day 38 |
| 14 | Day 47 (20 days) |
| 21 | Day 72 |
| 24 | Day 53 |
| 35 | Day 68 |
| 46 | Day 49 |
| 48 | Day 25 |
| 50 | Day 42 |
| 62 | Day 44 |
| 94 | Day 36 |
| 96 | Day 77 |
| 97 | Day 39 |
| 98 | Day 53 |
| 103 | Day 38 |
Table 1a: Increased from 1 to 2 lozenges (morning and afternoon doses).
| Participant no | Increased from 1 to 2 lozenges (night time dose) |
|---|---|
| 16 | Day 25 |
| 19 | Day 10 |
| 55 | Day 23 |
| 61 | Day 28 |
| 67 | Day 23 |
| 75 | Day 27 |
| 77 | Day 8 |
| 82 | Day 60 |
| 86 | Day 12 |
| 90 | Day 45 |
| 101 | Day 44 |
Table 1b: Increased from 1 to 2 lozenges (night time dose).
| Participant no. | Increased all 3 lozenges from 1 to 2 |
|---|---|
| 23 | Day 59 |
| 26 | Day 62 |
| 42 | Day 72 |
| 44 | Day 67 |
| 63 | Day 67 |
| 69 | Day 41 |
| 77 | Day 36 |
| 89 | Day 55 |
Table 1c: Increased all 3 lozenges from 1 to 2.
Discussion
Vasomotor Symptoms (VMS), including hot flashes, flushes and night sweats, are a primary symptom of menopausal women; given the prevalence and duration of VMS, an alternative treatment to hormonal and non-hormonal therapies and supplements was developed via a lozenge supplement system. A series of case studies demonstrated an efficient delivery system of active ingredients and a positive trend toward an improvement in menopausal symptoms among women [3-8]. this clinical study was conducted to determine the efficacy and safety of the proprietary Directline® delivery method utilized in the Rebalance Health supplement system for the reduction of vasomotor symptoms in women. Participants experienced a statistically significant decrease in hot flashes, which continued to decline over the 12-week study. As the participants in the study had varying frequencies of hot flashes, the data was segmented into 4 groups and further analysis revealed that those who experienced an average of 2-4 hot flashes per day at baseline had a 76% reduction in hot flashes by week 12. Similarly, baseline participants with 5-7 hot flashes experienced a 78% reduction by week 12. Those with 8-10 hot flashes at baseline had a 74% reduction, while those with 11 or more hot flashes experienced an 83% reduction by the end of week 12. These results align with the overall mean change of an 80% reduction in hot flash frequency.
Additionally, a majority of participants experienced significant improvements related to their other menopausal symptoms. Specifically, 86% of the participants reported improved disposition and mood, while 85% noticed a more consistent sleep and felt rested upon waking in the morning. Moreover, 78% of the participants experienced an improvement in their focus, alertness and reduced brain fog.
A possible limitation or alternative for this study design would be to include a placebo group and collect and analyze blood serum samples from a larger sample size. Most participants were enrolled during the summer months when extreme heat has a tendency to escalate VMS. Some participants did experience this escalation, but those time points were still statistically significant compared to baseline.
Women who are precluded from using hormonal or nonhormonal prescription therapies may benefit from the use of this supplement system in the management of hot flashes and other menopausal symptoms.
Conclusion
A supplement system using a proprietary Directline® delivery method via a lozenge demonstrated a significant decrease in vasomotor symptoms over a 12-week study. Additionally, women reported improvements in their mood, disposition, sleep, stress response, and cognitive function. This supplement system is a viable alternative to hormonal or non-hormonal therapies for mitigating vasomotor symptoms in menopausal women.
Author Contribution
Todd Dorfman: Owner and Medical Director of Cedalion Health, Boulder, CO. Chief Medical Officer Rebalance Health, Inc.,; Amber Krogsrud: Owner of Peptide RX, West Palm Beach, FL. Specializes in women’s health, hormone testing and treatment, regenerative medicine, and the use of therapeutic peptides; Alison Gracom: Owner of Complete Care Medical Group, Inc., Irvine, CA. Expert lecturer on the topic of cortisol; Russell Glenn: Vice President of Product Development Rebalance Health Inc.,; Justin Hai: President and CEO Rebalance Health Inc.,
References
- National Institute of Aging (2023) Research explores the impact of menopause on women’s health and aging.
- Paciuc J (2020) Hormone therapy in menopause. Adv Exp Med Biol 1242: 89-120.
[Crossref], [Google Scholar], [Indexed]
- Dorfman T, Krogsrud A, Gracom A (2023) A case study of a novel nutraceutical with a proprietary delivery system. Vitam Miner 12: 278.
[Crossref]
- Duralde ER, Sobel TH, Manson JE (2023) Management of perimenopausal and menopausal symptoms. BMJ 382: e072612.
[Crossref], [Google Scholar], [Indexed]
- Crandall CJ, Mehta JM, Manson JE (2023) Management of menopausal symptoms: A review. JAMA 329: 405-420.
[Crossref], [Google Scholar], [Indexed]
- OlaOlorun F, Shen W (2020) Menopause. Global Public Health.
[Crossref]
- Hariri L, Rehman A (2023) Estradiol. StatPearls Publishing: Florida, USA.
- Thurston RC, Joffe H (2011) Vasomotor symptoms and menopause: Findings from the study of women's health across the nation. Obstet Gynecol Clin North Am 38: 489-501.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences