Osmo-Dehydration of Strawberries Coated with Arabic Gum using Response Surface Optimization
Roshanak Rezaei, Mirkhalil Pirouzifard and Mohammad Alizadeh Khaled-Abad
Roshanak Rezaei, Mirkhalil Pirouzifard and Mohammad Alizadeh Khaled-Abad*
Faculty of Agricultural Engineering, Department of Food Science and Technology, University of Urmia, Iran
- *Corresponding Author:
- Mohammad Alizadeh Khaled-Abad
Faculty of Agricultural Engineering, Department of Food Science and Technology, University of Urmia, Iran.
Tel: +989143474341
E-mail: m.alizadeh7@gmail.com
Received Date: December 18, 2017; Accepted Date: January 17, 2018; Published Date: January 30, 2018
Citation: Rezaei R, Pirouzifard M, Khaled- Abad MA (2018) Osmo-Dehydration of Strawberries Coated with Arabic Gum using Response Surface Optimization. J Nutraceuticals Food Sci. Vol.3 No.1:2.
Abstract
The aim of present study was to optimize the osmo-dehydration process condition of strawberries coated with Arabic gum. The dependent variables were osmotic agent concentration (40% to 60% w/w), immersion time (12-48 h), coating concentration (0% to 2% w/w) and storage duration (0-4 months). The experiments were designed according to central composite design and osmo-air dehydrated strawberries were evaluated for density, water content, colorimetric parameters, total anthocyanin content and antioxidant activity. It was found that Arabic gum effectively contributed in retention of colour and nutrient content while it showed significantly negative effect on water content (p<0.05). Elongation of process duration and storage time led to major losses (p<0.01) of product quality. Response surface methodology (RSM) revealed that osmo-dehydration of strawberries coated with 1.3% Arabic gum in sucrose solution of 50.64% w/w concentration for 12 h would give best performance in term of nutrient content and colour retention as well as product humidity and density over 67 days of storage time.
Keywords
Strawberry; Arabic gum; Osmo-dehydration; Response surface methodology
Introduction
Strawberries (Fragaria vesca L.) have a unique aromatic taste and prosperity of cherished nutrients [1]. The nutritional qualities of the berries are ascribed to the presence of phenolic compounds. Although not essential for human life, phenolic compounds may act as a health promoting factor, if consumed over a long period of time [2]. It is noteworthy that phenolic compounds demonstrated a suitable antioxidant and antimicrobial properties that nominate them as supportive to the natural defences of the human body and reducing the risk of certain cancers and cardiovascular diseases [3]. Strawberries rich in different phenolic compounds including ellagic acid, ellagic acid glycosides, p-coumaric acid derivatives, ellagitannins, gallotannins, anthocyanins, flavanols, flavanols and coumaroyl glycosides. The anthocyanidins were pelargonidin and cyanidin, found chiefly as their glucosides and rutinosides forms. The major flavanol aglycons were quercetin and kaempferol found as their glucuronides and glucosides [4,5]. Because of the seasonality and delicate texture, fresh strawberries are available only for a few months a year.
One of the approaches of extending the postharvest life of strawberries is osmotic dehydration, which also makes it possible to alter the composition of the hydration raw materials. The process consists in submerging hydration raw materials of cellular structure in a hypertonic solution [6]. During the process, the water existing in the tissues is liberated to the solution and mass transfer occurred between the solution and tissue components. Generated gradient of osmotic pressure between the osmotic solution and the vacuolar sap of the fresh raw materials exposed to dehydration is the main driving force of the process at low temperatures and short processing times. Water and substances from the sap are exchanged through the plant material semipermeable cell membrane [2]. It is observed that the osmotic dehydration process decreases the amount of initial water content to about 50%, reduces the weight and volume of the product and improves the organoleptic features of the finished products. Additionally, the lack of oxygen during the process can hinder the oxidation and enzymatic browning of plant constitutes [7]. Diffusion phenomena are responsible for controlling the transfers at higher temperatures and long process times. Accordingly, unfavorable changes comprising the loss of semi-permeability of cell membranes, high sugar incorporation and substantial losses in valuable nutrients may achieved due to the inadequate handling of dehydration parameters in the dehydrated material [8]. So, the employing osmotic dehydration can result in substantial quality improvement and considerable economic benefits, due to increased product competitiveness compared to alternative processes.
Osmotic treatment can be used as a major pre-treatment prior to a wide range of processing schemes, including whole drying, pasteurization and freezing. Osmotic dehydration commonly includes substantial water removal (40% to 70% loss of initial moisture) with much lower uptake of osmotic solute, largely depending on osmotic solute and process conditions. Abundant solute uptake is considered undesirable for osmotic dehydration purposes, because of adverse impact on the natural product profile. Thus, many researches have focused on techniques to monitor solute uptake [9,10]. Former efforts have presented that, solute penetration mainly depends on solute molecular size and process parameters, comprising solution concentration and process temperature. Pre-coating the plant goods to be dehydrated with an artificial, edible barrier was also found as a way to efficiently hamper solute penetration, without a serious undesirable impact on the water removal rate [11].
Edible coatings mainly originated from lipids, polysaccharides, resins and proteins and also a mixture of these materials forms the new composite edible coatings which can limit lipid, oxygen, water vapor and flavor migration between food and the surroundings. Low-methoxyl pectinate, high-methoxyl pectinate, methyl cellulose, carboxyl methyl cellulose, maltodextrin, potato starch, corn starch, sodium alginate, chitosan and different gums can be employed as coating solutions to prevent solid gain and improve organoleptic properties, shelf-life and nutritive properties of vegetables and fruits during osmotic dehydration [12].
One of the gums that exploited as a coating agent is Arabic gum which widely used as an additive in food materials e.g. confectionery, ice-cream industries and bakery products. It is classified as an edible coating and it is used to increase stability and shelf-life by increasing the cell wall thickness and to enhance microbial safety of fruits.
The objective of this study was to optimize the influence of sucrose solution and Arabic gum pre-treatments on physicochemical properties and bioactive compounds alteration of osmo-hydrated strawberries during the storage which Arabic gum was not used to coating the osmo-hydrated strawberries till now.
Materials and Methods
Chemicals and reagents
Fresh strawberries cv. Kurdistan acquired from a local market in Sanandaj (Kurdistan, Iran) and stored at 30°C for two weeks before testing. Absolute methanol and 2,2-diphenyl-1picrylhydrazyl (DPPH) reagent were purchased from Merck (Darmstadt, Germany). Also, Arabic gum and citric acid were obtained from Scharlau Co. (Barcelona, Spain). All other chemicals used in this study were of analytical grade quality.
Preparation of osmotic solutions
The ingredients of prepared osmotic solutions were sucrose, Arabic gum, citric acid and distilled water. Calculating the concentrations was based on w/w. The solutions variables were the Arabic gum and sucrose concentrations, while the amount of citric acid (2%) and distilled water were constant. The fruit to osmotic solution ratio set at 1:5 in all experiments.
Osmotic dehydration and hot-air drying
Strawberry slices poured into plastic osmotic solution container after weighting so that slices immersed in the solution. During the immersion, the mixture is stirred periodically (every 2 hours for 5 min) to obtain uniform product. Dehydration process was conducted at constant temperature of incubator (30°C). After immersion, strawberry slices removed from osmotic solution and submerged in cold water to eliminate surface sucrose. Then, surface moisture removed with special filter papers, now samples are ready to transfer to hot-air dryer (at 30°C 122 for 8 h; with 1.2 m/s rate). Finally, osmo-hydrated slices were cooled for 10 min at room temperature, packaged in polyethylene bags and maintained in a dry place to carry out various experiments.
Physical parameters
Water content was determined first by oven-drying of the samples at 105°C for 48 h. for calculating the pH value, the samples were thoroughly uniformed with a mixer and pH were recorded by pHmeter (Modell A420, Orion, USA). In order to evaluate the density of samples, 100 strawberry fruits were randomly selected and weighted, then their apparent density were calculated as follow:
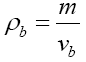
where m and vb are the mass and volume of the selected samples, respectively.
Surface colour assessment
A colorimeter apparatus (Minolta CR 400 Series, Osaka, Japan) was employed to determine the colour of the strawberries. Previously, the apparatus was adjusted using a standard white plate and standard colour parameters (L′= 84.71, a′=+1.26, b′=−3.5). Then, the samples were 139 positioned on a standard white plate and colour parameters were determined. The calculated 140 parameters were L (lightness), a (red-green) and b (blue-yellow). Hence, total colour variation from standard (ΔE), browning index (BI), hue angle (H*) and chroma (ΔC or C*) 142 were calculated according to the following equations [13]:
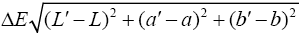
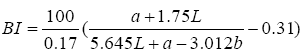
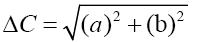
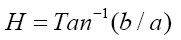
The results were expressed as the average of three measurements from five points of each sample.
Antioxidant activity
DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging capacity of the extracts was evaluated according to Garavand et al, with some modifications [14]. Briefly, after being diluted in distilled water (1:10), 0.1 mL of the extract was mixed with 3.9 mL methanolic DPPH 154 solution (25 mgL-1). The mixture was vortexed severely and left to stand for 30 min at room temperature. A DPPH solution with no added extract was considered as the control. The inhibition percentage of DPPH was measured at 517 nm according to the following equation:
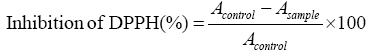
Where Asample and Acontrol are the absorbance of the extract sample with DPPH and the absorbance of the DPPH solution without extract, respectively.
Determination of total anthocyanin content (TAC)
The total anthocyanin content of the strawberries was determined by the pH differential method which is a spectrophotometric assay that involves absorbance measurement of extracts at pH 1.0 and 4.5 [15]. The absorbance of the samples was determined at 520 and 700 nm using spectrophotometer and total anthocyanin content (mg/L) was stated as cyanidin-3-glucoside according to the following equation:
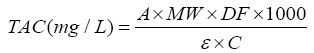
where A is absorbance of samples and calculated as follow:
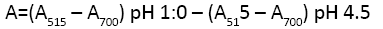
MW is molecular weight of malvidin-3-glucoside=449.2 g/mol; DF is dilution factor, ε is 171 molar absorptivity of cyanidin-3- O-glucoside=26,900; and C is the concentration of the buffer in mg/ml. The obtained results were represented as mg cyanidin- 3-glucoside 173 equivalents/g Dry sample (mg c-3-gE/Kg Dry matter).
Experimental design and statistical analysis
The optimum osmo-dehydration process condition, coating concentration and storage time 177 for strawberries were determined according to a statistical design by RSM. The independent variables were osmotic agent concentration, immersion time, coating concentration and storage duration. Responses were density, water content, colorimetric parameters (L*, C* and H*), total anthocyanin content and antioxidant activity. A central composite design (CCD) with four factors at five levels was performed to generate 30 runs. Coded and decoded settings of the process parameters are presented in Table 1. The design comprised of 16 factorial points, 6 replicates of the central point and 8 axial points. Factorial points were employed to estimate the linear and interaction effects while center points provide the possibility of checking for curvature in response and axial points are used to estimate the quadratic terms [16]. A second-order polynomial equation was fitted to the experimental data of responses:
| Dependent variables | Level | ||||
|---|---|---|---|---|---|
| -2 | -1 | 0 | 1 | 2 | |
| X1: Sucrose concentration as osmotic agent (g/100ml) | 40 | 45 | 50 | 55 | 60 |
| X2: immersion time (h) | 12 | 21 | 30 | 39 | 48 |
| X3: gum Arabic concentration as coating material (g/100ml) | 0 | 0.5 | 1 | 1.5 | 2 |
| X4: storage time (month) | 0 | 1 | 2 | 3 | 4 |
Table 1: Coded and decoded levels of the process parameters for osmo-dehydration of 5 strawberries based on a central composite design.
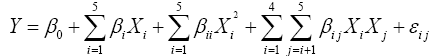
where Y is the response, β0, βi, βii and βij are coefficients for intercept, linear, quadratic and 190 interaction coefficients, respectively and Xi and Xj are the independent variables. Minitab 15 Software was used for response surface analysis, plotting the graphs as well as response optimization.
Results and Discussion
Water content and density
The effects of process variables on water content and density of coated strawberries during and after osmotic dehydration are presented in Table 2. It is evident from the table that the density of osmotically dehydrated strawberries was remarkably (p<0.01) dependent on osmotic solution concentration while it only showed a negligible negative effect on their water content. It is believed that surrounding the fresh fruit by a highly concentrated solution during osmotic dehydration provides strong driving force between hypertonic solution and fruit tissue for mass transfer of fruit water into the solution and osmotic agent into the fruit tissue which in turn may lead to a denser dehydrated fruit [17]. There have been many researches on increased water loss and solid gain of osmo-dehydrated fruits with increasing the osmotic solution concentration [18-20]. However, it has been pointed out that increasing the sugar concentration beyond a certain level reduced the water loss attributed to crystallization of sugar at high solution concentration which prevented moisture removal from the fruit [21]. The results indicated that there would be drastic increase in density (p<0.01) and a decreased water content (p<0.05) as osmotic dehydration process progressed (Table 2). It has been found by some researchers that most of sucrose gain and water loss of strawberries took place in initial hours of immersion time [22]. This trend has also been observed by other researchers during osmo-dehydration of potato, pineapple, carrot, cucumber and so on [18,19,23,24]. This might be as a result of reaching equilibrium between cellular fluids and osmotic solution at longer process duration [21]. As deduced from the Table 2, the strawberries coated with higher concentration of Arabic gum significantly (p<0.01) showed resistance to water loss and sugar gain. This is in line with the results of Khin et al. who observed that coating the apples with hydrophilic coating materials considerably decreased the water loss during osmotic dehydration over low process temperatures [25]. However, coating has been shown as an effective pre-treatment strategy to hinder sugar penetration into the food without a serious negative effect on water loss [11,26]. These differences between barrier properties of different coatings can be interpreted through their compositions and the methods used for their fabrication [11].
| Source | DF | Sum of Squares | Mean Square | F-value | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y1 | Y2 | Y1 | Y2 | Y1 | Y2 | ||
| Regression | 14 | 0.123 | 26.31 | 0.008 | 1.87 | 9.78 | 12.1 | 0 | 0 |
| Linear | 4 | 0.099 | 17.53 | 0.024 | 4.38 | 27.58 | 28.22 | 0 | 0 |
| Quadratic | 4 | 0.014 | 7.07 | 0.003 | 1.76 | 4.05 | 11.38 | 0.02 | 0 |
| Interaction | 6 | 0.009 | 1.71 | 0.001 | 0.28 | 1.72 | 1.84 | 0.184 | 0.159 |
| Residual Errors | 15 | 0.013 | 2.32 | 0 | 0.15 | - | - | - | - |
| Lack-of-Fit | 10 | 0.011 | 1.63 | 0.001 | 0.16 | 2.88 | 1.17 | 0.128 | 0.457 |
| Pure Error | 5 | 0.002 | 0.69 | 0 | 0.13 | - | - | - | - |
| Total | 29 | 0.136 | 28.64 | - | - | - | - | - | - |
| Other statistics | Y1 | Y2 | |||||||
| Source | b-coefficient | p-value | b-coefficient | p-value | |||||
| Intercept | 1.37 | 0 | 18.048 | 0 | |||||
| X1 | 0.09 | 0 | -0.105 | 0.524 | |||||
| X2 | 0.08 | 0 | -0.398 | 0.026 | |||||
| X3 | -0.045 | 0.012 | 1.115 | 0 | |||||
| X4 | 0.005 | 0.689 | -1.228 | 0 | |||||
| X1. X1 | -0.06 | 0.019 | 0.142 | 0.643 | |||||
| X2. X2 | -0.07 | 0.008 | 0.442 | 0.162 | |||||
| X3. X3 | -0.04 | 0.101 | 0.627 | 0.055 | |||||
| X4. X4 | -0.04 | 0.101 | 1.987 | 0 | |||||
| X1. X2 | 0.05 | 0.116 | -0.085 | 0.832 | |||||
| X1. X3 | 0.075 | 0.025 | -0.06 | 0.881 | |||||
| X1. X4 | -0.025 | 0.418 | 0.46 | 0.261 | |||||
| X2. X3 | -0.015 | 0.624 | -0.28 | 0.448 | |||||
| X2. X4 | 0.015 | 0.624 | 0.17 | 0.672 | |||||
| X3. X4 | 0.01 | 0.743 | 1.175 | 0.009 | |||||
| R2 | 0.901 | - | 0.919 | - | |||||
| R2-adjust | 0.809 | - | 0.843 | - | |||||
| X1, X2, X3 and X4 are osmotic agent concentration, immersion time, coating concentration and storage time, respectively | |||||||||
Table 2: Analysis of variance (ANOVA) 33 for density (Y1) and water content (Y2).
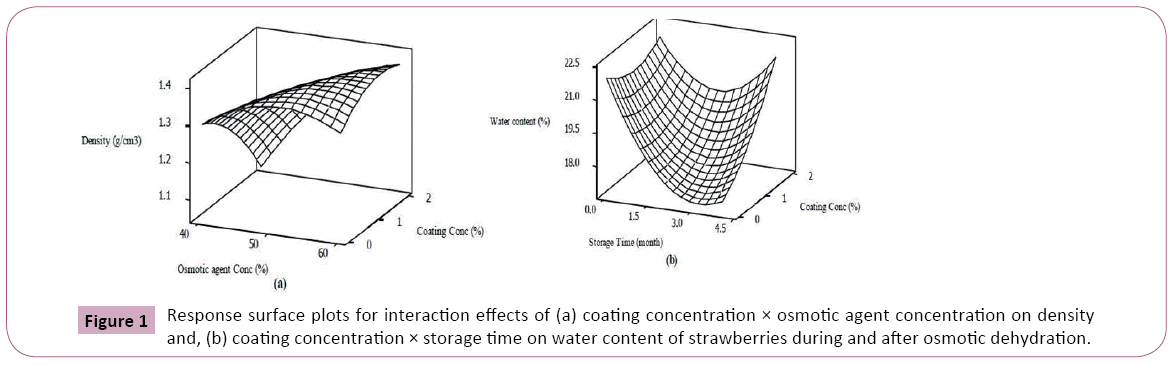
The interaction effect of coating concentration × osmotic agent concentration on density is depicted by response surface plot (Figure 1). Model equations are visualized in the form of threedimensional surface plots which are constructed by plotting the dependent variable on the Z-axis versus any two independent variables while the other variable is set on the middle level [16]. As can be seen, at lower osmotic solution concentration, the strawberries treated with higher concentration of Arabic gum were more resistant against sugar uptake and thus increasing density whereas, the rate of sugar gaining and increasing the density was promoted when osmo-dehydration was performed in more concentrated osmotic solution. From the response surface graph (Figures 1a and 1b), at initial days of storage, coating showed higher water permeability possibly due to larger driving force for mass transfer of water but the longer the storage time, the more effective were coatings against humidity reduction. In consistent with our results, Castell et al. reported notable water lost for non-coated osmo-dehydrated strawberries during storage. On the other hand, Garcia et al indicated that coating the minimally processed strawberries with cassava starch enhanced their resistance to water vapor during storage [27,28].
Colour changes
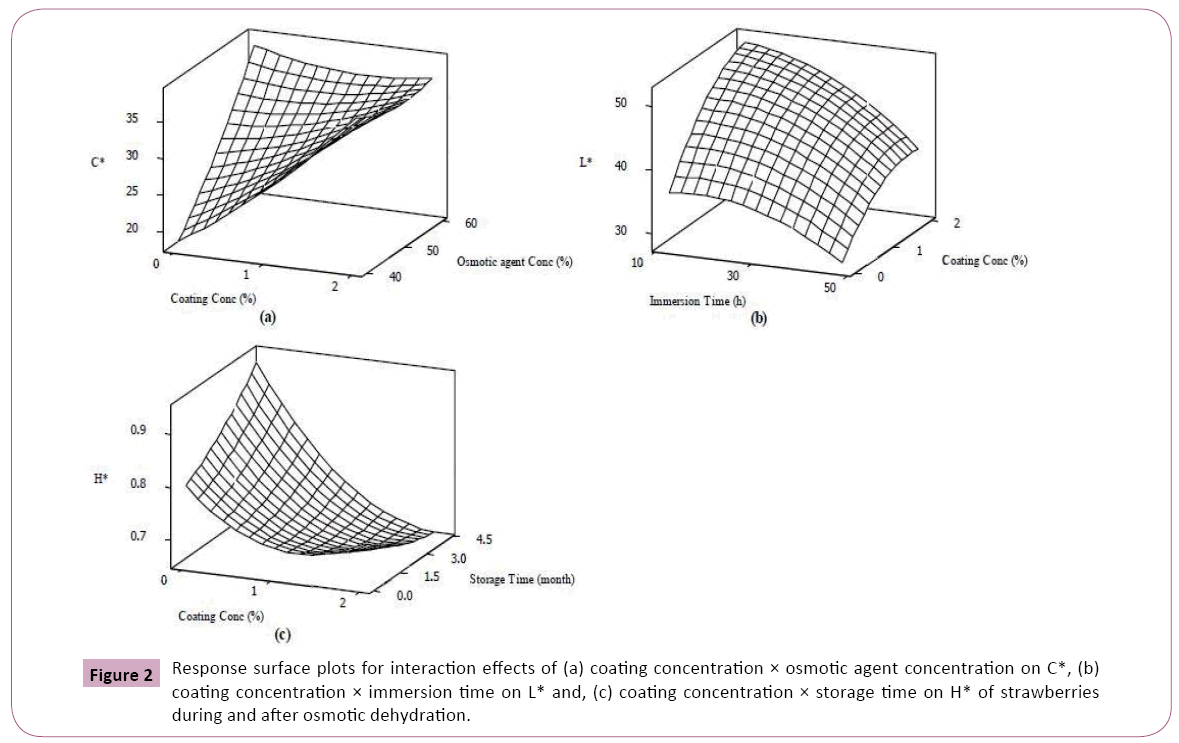
The results revealed that strawberries osmotically dehydrated in higher concentration of sucrose solution showed better performance in term of colour retention (Table 3). As presented in the Table 3, there is a significant enhancement in lightness and C* value with osmotic agent concentration (p<0.01). Similarly, it was reported that an increase in redness of carrot cubes dehydrated in more concentrated osmotic solutions [20]. It is thought that increased diffusion of osmotic agent into the fruit cell at higher solution concentration (Table 2) might cause disruption of plasma membranes and cell walls resulted in spreading out the carotenoids, responsible for redness and yellowness of fruits, throughout the cell which in turn might lead to higher C* value [29]. Figures 2a-2c shows that rising trend of C* with increase of osmotic solution concentration experienced a reduction as coating concentration was promoted possibly due to barrier effect of coating against sugar uptake.
| Source | DF | Sum of Squares | Mean Square | F-value | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | C* | H* | L* | C* | H* | L* | C* | H* | L* | C* | H* | ||
| Regression | 14 | 754.51 | 352.95 | 0.067 | 53.89 | 25.21 | 0.0048 | 6.68 | 20.75 | 9.55 | 0 | 0 | 0 |
| Linear | 4 | 600.39 | 309.64 | 0.054 | 150.09 | 77.41 | 0.0135 | 18.62 | 63.7 | 26.96 | 0 | 0 | 0 |
| Quadratic | 4 | 69.97 | 9.14 | 0.009 | 17.49 | 2.28 | 0.0023 | 2.17 | 1.88 | 4.6 | 0.122 | 0.166 | 0.013 |
| Interaction | 6 | 84.16 | 34.16 | 0.003 | 14.02 | 5.69 | 0.0006 | 1.74 | 4.69 | 1.24 | 0.18 | 0.007 | 0.341 |
| Residual Errors | 15 | 120.95 | 18.22 | 0.007 | 8.06 | 1.21 | 0.0005 | - | - | - | - | - | - |
| Lack-of-Fit | 10 | 103.45 | 8.32 | 0.005 | 10.34 | 0.83 | 0.0005 | 2.96 | 0.42 | 1.1 | 0.122 | 0.886 | 0.486 |
| Pure Error | 5 | 17.49 | 9.9 | 0.002 | 3.49 | 1.98 | 0.0004 | - | - | - | - | - | - |
| Total | 29 | 875.46 | 371.18 | 0.074 | - | - | - | - | - | - | - | - | - |
| Other statistics | L* | C* | H* | ||||||||||
| Source | b-coefficient | p-value | b-coefficient | p-value | b-coefficient | p-value | |||||||
| Intercept | 44.5767 | 0 | 30.1667 | 0 | 0.597 | 0 | |||||||
| X1 | 5.0558 | 0.001 | 4.4383 | 0 | 0.017 | 0.075 | |||||||
| X2 | 5.0575 | 0.001 | 2.3483 | 0.012 | 0.032 | 0.003 | |||||||
| X3 | 6.4958 | 0 | 4.6283 | 0 | 0.085 | 0 | |||||||
| X4 | 2.5942 | 0.041 | 2.23 | 0 | 0.017 | 0.075 | |||||||
| X1. X1 | 4.9821 | 0.036 | 1.3758 | 0.123 | 0.039 | 0.035 | |||||||
| X2. X2 | 1.7371 | 0.436 | 1.6208 | 0.073 | 0.029 | 0.105 | |||||||
| X3. X3 | 2.0921 | 0.35 | 1.3808 | 0.122 | 0.064 | 0.002 | |||||||
| X4. X4 | 4.5371 | 0.054 | 0.0642 | 0.94 | 0.014 | 0.408 | |||||||
| X1. X2 | 4.9225 | 0.103 | 0.13 | 0.908 | 0.007 | 0.743 | |||||||
| X1. X3 | 6.1875 | 0.066 | 5.38 | 0 | 0.017 | 0.447 | |||||||
| X1. X4 | 0.3225 | 0.911 | 0.08 | 0.943 | 0.017 | 0.447 | |||||||
| X2. X3 | 0.9275 | 0.049 | 1.185 | 0.299 | 0.017 | 0.447 | |||||||
| X2. X4 | 0.9225 | 0.75 | 1.855 | 0.113 | 0.002 | 0.913 | |||||||
| X3. X4 | 4.4525 | 0.138 | 0.595 | 0.597 | 0.052 | 0.033 | |||||||
| R2 | 0.862 | 0.951 | 0.899 | ||||||||||
| R2-adjust | 0.733 | 0.905 | 0.805 | ||||||||||
| X1, X2, X3 and X4 are osmotic agent concentration, immersion time, coating concentration and storage time, respectively | |||||||||||||
Table 3: Analysis of variance 46 (ANOVA) for colorimetric parameters.
In disagreement with our results, in an effort to study the process variables on colour retention of cherry tomato during osmotic dehydration found a decreased lightness over higher osmotic solution concentration [29]. Our observation on increasing the L* with osmotic solution could be explained by limited enzymatic browning reaction at higher osmotic solution concentration [13]. The results indicated a strong positive relationship between process duration and H* value (p<0.01) while L* (p<0.01) and C* (p<0.05) values were affected negatively as osmotic dehydration progressed (Table 3). Similar results were reported by other researchers and [20,29]. The reducing effect of process length on food lightness during osmotic dehydration seems to be mainly because of shrinkage of cellular structure due to water loss which consequently resulted in sample opacity [29]. Likewise, less saturation must be responsible for changing the vivid colour of fresh strawberries to the pale appearance (lower C* and higher H*) of the dehydrated samples [30]. The deceased lightness might be as well arisen from increased oxygen solubility at longer immersion time which might promote enzymatic browning [13]. A better colour retention has been reported for osmo-dehydrated samples when a blanching step was carried out before process [31,32]. It is important to mention that decreasing effect of process duration on lightness was less strong for strawberries coated with higher concentration of Arabic gum (Figure 2b). The beneficial effect of coating on colour retention of strawberries can also be observed during storage (Figure 2c). The osmo-dehydrated samples tended to show larger H* during storage but at higher coating concentration, their tendency was affected negatively it was observed that there was severe decrease in L* and b* of osmo-dehydrated seaweed over shelf life [32]. As shown in Table 2, elongation of storage time resulted in a significant reduction in C* and L* of dehydrated strawberries. This could be closely related to microstructure modification of strawberries during mass exchange of osmotic dehydration which might damage fragile membrane of carotenoids facilitating their oxidative or enzymatic degradation over storage [33]. However, scanning electron microscopy results of some researchers indicated that cellular structure of the coated apples was better maintained compared to the non-coated samples against mechanical damages during osmotic dehydration [25].
Total anthocyanin content and total antioxidant activity
Data showed that fresh strawberries experienced substantial losses of 53.28-74.69% in total anthocyanin compounds during osmotic dehydration and shelf life concluded that anthocyanin concentrations of dehydrated blueberries are significantly lower in those osmotically pre-treated compared to untreated ones [2]. Changes in anthocyanin content and antioxidant activity of strawberries followed the same pattern Table 4 as the antioxidant properties in berries mainly come from anthocyanins and phenolics [34].
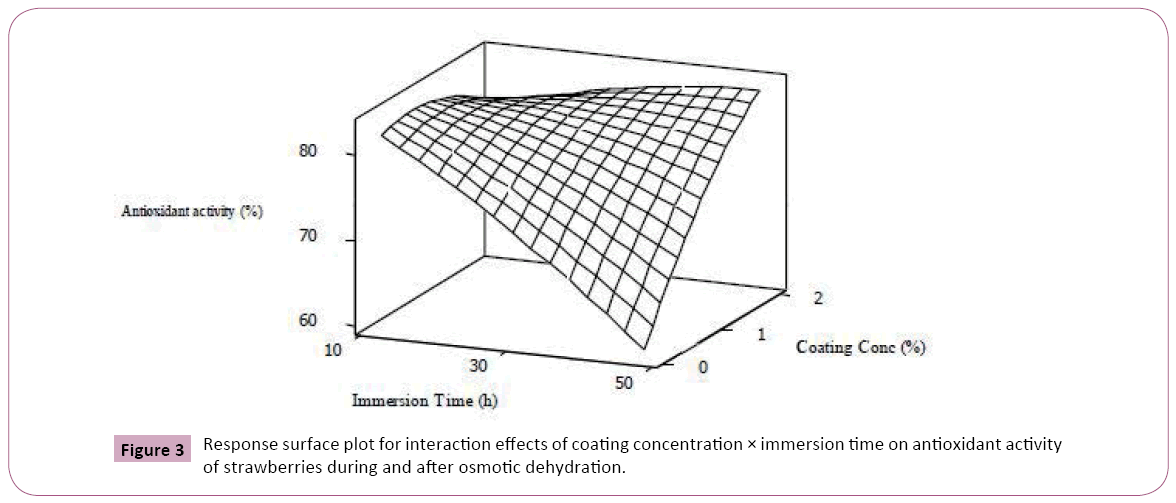
Statistical analysis revealed that immersion time had the most significant negative effect (p<0.01) on the total anthocyanin content and antioxidant activity. observed an approximately 60% losses in anthocyanins and phenolics for blueberries osmoconcentrated in sucrose solution for 12 hours. This phenomenon is mostly related to migration of anthocyanin compounds to dehydrating solution along with water transfer [7,34]. As implied by Figure 3, increasing the coating concentration could effectively prevent anthocyanin leakage into the osmotic solution.
Our results indicated that storage duration had the same effect on anthocyanin content and antioxidant activity of strawberries as immersion time (Table 4). Significant reduction of anthocyanin during storing has previously been reported for cherries [35].
Optimization
Optimization of the osmotic dehydration condition for strawberries was conducted to reach maximum colour retention and nutritional quality and minimum density and humidity during dehydration process as well as storage time. The optimum conditions yielded by RSM are presented in Table 5. The performance of models in predicting the optimum condition was verified using one-sample t-test. The strawberries were osmotically dehydrated under optimum condition and the responses of interest were determined at the end of the desire storage time. The results of one-sample t-test confirmed that predicted and measured characteristics of osmo-dehydrated strawberries did not statistically differ with a 95% of confidential level.
Conclusion
The osmotic dehydration process can be employed for prolonging the shelf-life of food products for the purpose of use during offseason. Our results revealed that despite having negative effect on water loss, Arabic gum played a decisive role in retention of colour and nutrient content of strawberries not only during osmotic dehydration but also over shelf life. So, the osmodehydrated strawberries in this study have a good capability to be used as preserves, fruit juice, pies, ice creams, milkshakes, and chocolates.
References
- Nuñez-Mancilla Y, Pérez-Won M, Uribe E, Vega-Gálvez A, Di Scala K (2013) Osmotic dehydration under high hydrostatic pressure: Effects on antioxidant activity, total phenolics compounds, vitamin C and colour of strawberry (Fragariavesca). LWT-Food Sci Technol 52: 151-156.
- Mirzaee S, Askari GR, Z Emam-Djomeh, Garavand F (2016) Changes in bioactive compounds, quality attributes and rheological behaviour of black grape juice caused by microwave and conventional heating. Nutrafoods 15: 285-292.
- Garavand F, Madadlou A (2014) Recovery of phenolic compounds from effluents by a microemulsion liquid membrane (MLM) extractor. Colloids Surf PhysicochemEng Asp 443: 303-310.
- Seeram NP, Lee R, Scheuller HS, Heber D (2006) Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem 97: 1-11.
- Aaby K, Ekeberg D, Skrede G (2007) Characterization of phenolic compounds in strawberry (Fragariaananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J Agri Food Chem 55: 4395-4406.
- Mavroudis NE, Dejmek P, Sjöholm I (2004) Studies on some raw material characteristics in different Swedish apple varieties. J Food Eng 62: 121-129.
- Ketata M, Desjardins Y, Ratti C (2013) Effect of liquid nitrogen pre-treatments on osmotic dehydration of blueberries. J Food Eng 116: 202-212.
- Falade KO, Igbeka JC (2007) Osmotic dehydration of tropical fruits and vegetables. Food Rev Int 23: 373-405.
- Khin MM, Zhou W, Perera CO (2006) A study of the mass transfer in osmotic dehydration of coated potato cubes. J Food Eng 77: 84-95.
- Akbarian M, Ghanbarzadeh B, Sowti M, Dehghannya J (2015) Effects of pectin CMC based coating and osmotic dehydration pretreatments on microstructure and texture of the hot air-dried quince slices. J Food Process Preserv 39: 260-269.
- Matuska M, Lenart A, Lazarides HN (2006) On the use of edible coatings to monitor osmotic dehydration kinetics for minimal solids uptake. J Food Eng 72: 85-91.
- Bonilla J, Atarés L, Vargas M, Chiralt A (2012) Edible films and coatings to prevent the detrimental effect of oxygen on food quality: possibilities and limitations. J Food Eng 110: 208-213.
- Jalaee F, Fazeli A, Fatemian H, Tavakolipour H (2011) Mass transfer coefficient and the characteristics of coated apples in osmotic dehydrating. Food Bioprod Process 89: 367-374.
- Garavand F, Madadlou A, Moini S (2015) Determination of phenolic profile and antioxidant activity of pistachio hull using high-performance liquid chromatography-diode array detector-electro-spray ionization-mass spectrometry as affected by ultrasound and microwave. Int J Food Prop 20: 19-29.
- Garavand F, Madadlou A (2013) Extraction of phenolic compounds from pistachio processing unit effluent by developed microemulsion liquid membrane as a nanocareer. Electron J Food Process Preserv 5: 61-79.
- Goudarzi M, Madadlou A, Mousavi ME, Emam-Djomeh Z (2012) Optimized preparation of ACE-inhibitory and antioxidative whey protein hydrolysate using response surface method (RSM). Dairy Sci Technol 92: 641-653.
- El-Aouar AA, Azoubel PM, Barbosa JJL, Murr FEX (2006) Influence of the osmotic agent on the osmotic dehydration of papaya (Carica Papaya L.). J Food Eng 75: 267-274.
- Saputra D (2001) Osmotic dehydration of pineapple. Dry Technol 19: 415-425.
- Eren S, Kaymak-Ertekin F (2007) Optimization of osmotic dehydration of potato using response surface methodology. J Food Eng 79: 344-352.
- Singh B, Panesar PS, Nanda V, Kennedy JF (2010) Optimization of osmotic dehydration process of carrot cubes in mixtures of sucrose and sodium chloride solutions. Food Chem 123: 590-600.
- Suresh Kumar P, Devi P (2011) Optimization of some process variables in mass transfer kinetics of osmotic dehydration of pineapple slices. Int Food Res J 18: 221-228.
- Erle U, Schubert H (2001) Combined osmotic and microwave-vacuum dehydration of apples and strawberries. J Food Eng 49: 193-199.
- Koprivica GB, MišljenoviÃÆââ¬Å¾Ãâââ¬Â¡ NM, LeviÃÆââ¬Å¾Ãâââ¬Â¡ LB, JevriÃÆââ¬Å¾Ãâââ¬Â¡ LR, FilipÃÆââ¬Å¾ÃâÃÂev BV (2010) Osmotic dehydration of carrot in sugar beet molasses: Mass transfer kinetics. Acta Period Technol 41: 47-55.
- Dermesonlouoglou EK, Pourgouri S, Taoukis PS (2008) Kinetic study of the effect of the osmotic dehydration pre-treatment to the shelf life of frozen cucumber. Innov Food Sci Emerg 9: 542-549.
- Khin MM, Zhou W, Perera CO (2007) Impact of process conditions and coatings on the dehydration efficiency and cellular structure of apple tissue during osmotic dehydration. J Food Eng 79: 817-827.
- Emam-Djomeh Z, Dehghannya J, Sotudehgharabagh R (2006) Assessment of osmotic process in combination with coating on effective diffusivities during drying of apple slices. Dry Technol 24: 1159-1164.
- Castell ML, Fito PJ, Chiralt A (2010) Changes in respiration rate and physical properties of strawberries due to osmotic dehydration and storage. J Food Eng 97: 64-71.
- Garcia LC, Pereira LM, Sarantópoulos CIGL, Hubinger MD (2010) Selection of an edible starch coating for minimally processed strawberry. Food Bioprocess Technol 3: 834-842.
- Heredia A, Peinado I, Barrera C, Andres Grau A (2009) Influence of process variables on colour changes, carotenoids retention and cellular tissue alteration of cherry tomato during osmotic dehydration. J Food Comp Anal 22: 285-294.
- de Bruijn J, Bórquez R (2014) Quality retention in strawberries dried by emerging dehydration methods. Food Res Int 63: 42-48.
- Escriche I, Chiralt A, Moreno J, Serra JA (2000) Influence of blanching-osmotic dehydration treatments on volatile fraction of strawberries. J Food Sci 65: 1105-1111.
- Rodriguez TV, Rojas AM, Campos CA, Gerschenson LN (2003) Effect of osmotic dehydration on the quality of air-dried Porphyra. LWT-Food Sci Technol 36: 415-422.
- CiurzyÃÆââ¬Â¦Ãâââ¬Å¾ska A, Lenart A, GrÃÆââ¬Å¾Ãâââ¢da KJ (2014) Effect of pre-treatment conditions on content and activity of water and colour of freeze-dried pumpkin. LWT-Food Sci Technol 59: 10751081.
- Stojanovic J, Silva JL (2007) Influence of osmotic concentration, continuous high frequency ultrasound and dehydration on antioxidants, colour and chemical properties of rabbit eye blueberries. Food Chem 101: 898-906.
- Forni E, Polesello A, Torreggiani D (1993) Changes in Anthocyanins in cherries (Prunus avium) during osmodehydration, pasteurization and storage. Food Chem 48: 295-299.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences