Optimizing Gut Ecosystem Dynamics: An Advanced Investigation into the Impact of a Multifaceted Probiotic Intervention on Gut Health and Immune Modulation
Ateeb Shaikh*, Haleema Yezdani, Pratik Saraogi and Sameer Baluni
Department of Diabetology, Fellowship in Intensive Care Medicine, Hyderabad, India
Published Date: 2024-02-05DOI10.36648/ipctn.9.1.42
Ateeb Shaikh*, Haleema Yezdani, Pratik Saraogi and Sameer Baluni
Department of Diabetology, Fellowship in Intensive Care Medicine, Hyderabad, India
- *Corresponding Author:
- Ateeb Shaikh
Department of Diabetology, Fellowship in Intensive Care Medicine, Hyderabad,
India,
E-mail: ateeb@actofit.com
Received date: January 04, 2024, Manuscript No. IPCTN-24-18543; Editor assigned date: January 08, 2024, PreQC No. IPCTN-24-18543 (PQ); Reviewed date: January 22, 2024, QC No. IPCTN-24-18543; Revised date: January 29, 2024, Manuscript No. IPCTN-24-18543 (R); Published date: February 05, 2024, DOI: 10.36648/ipctn.9.1.42
Citation: Shaikh A, Yezdani H, Saraogi P, Baluni S (2024) Optimizing Gut Ecosystem Dynamics: An Advanced Investigation into the Impact of a Multifaceted Probiotic Intervention on Gut Health and Immune Modulation. J Nutraceuticals Food Sci Vol.9 No.1: 42.
Abstract
This research endeavors to unravel the intricate interplay between the gut microbiota, immune function, and a novel comprehensive good bug supplement comprising prebiotics, probiotics, and postbiotics. In the realm of advanced microbiome modulation, this double-blind randomized controlled trial engaged 30 discerning participants, systematically divided into an intervention group receiving the multifaceted supplement and a meticulously matched control group. Rigorous evaluation tools, including the Gut Drybiosiv analysis, an IBS questionnaire, and precise measurements of Immunoglobulin E (IgE) levels, were employed to discern nuanced changes. Outcomes illuminate compelling enhancements in gut microbiota composition, a reduction in Irritable Bowel Syndrome (IBS) symptoms, and a commendable decline in IgE levels among the intervention cohort.
Keywords
Gut microbiota; Probiotics; Irritable Bowel Syndrome (IBS); Immune modulation; Prebiotics
Introduction
The vast and intricate milieu of the human gut microbiota, comprising a staggering array of microorganisms, stands as a linchpin in orchestrating physiological homeostasis. Acknowledging the pivotal role of gut dysbiosis in various health conditions, including gastrointestinal maladies and perturbations in immune function, the exploration of innovative interventions becomes imperative. The evolution of probiotics, characterized by live microorganisms conferring documented health benefits, has transgressed into a nuanced amalgamation with prebiotics and postbiotics, each contributing uniquely to sculpting a symbiotic gut ecosystem [1-7].
This study aspires to augment the scholarly tapestry concerning the therapeutic vistas of a comprehensive good bug supplement, fusing prebiotics, probiotics, and postbiotics into a synergistic blend. Within this paradigm, the intricate relationship between gut health and immune modulation is meticulously scrutinized, transcending conventional investigations. Previous research hints at the potential efficacy of interventions directed towards the gut microbiota in ameliorating conditions such as Irritable Bowel Syndrome (IBS) and shaping systemic immune responses. Nevertheless, the current study distinguishes itself through its comprehensive approach, addressing the triad of prebiotics, probiotics, and postbiotics, thereby offering a more holistic insight into the potential benefits inherent in orchestrating a finely-tuned gut ecosystem [8-13].
In this meticulously crafted randomized controlled trial, a cohort of 30 judiciously chosen participants became the focal point of an inquiry seeking to unveil the impact of the good bug supplement. The adoption of a double-blind methodology serves as a sentinel against biases, ensuring the veracity of outcomes. The primary objective encapsulates a nuanced exploration of the effects of the intervention on the intricate tapestry of gut microbiota composition, the manifestation of IBS symptoms, and the modulation of immunological parameters, epitomized by the quantification of IgE levels. Noteworthy is the all-encompassing nature of the intervention, addressing not merely probiotic augmentation but encapsulating the holistic spectrum of prebiotic nourishment and postbiotic metabolic byproducts. This amalgamation imparts a distinctive character to the study, advancing beyond the rudimentary and venturing into the avant-garde precincts of advanced microbiome modulation.
Methodology
Study design
A randomized controlled double-blind trial was employed to minimize bias and ensure the robustness of the study.
Participants
A total of 30 participants (aged 25-50 years) were recruited from local community/ medical center. Inclusion criteria included individuals without chronic medical conditions, not on antibiotics or probiotics for at least two months, and not diagnosed with severe gastrointestinal disorders.
Participants were randomly assigned to either the intervention group (receiving the good bug supplement) or the control group (receiving a placebo).
Intervention
The intervention group received a comprehensive good bug supplement twice daily for a duration of 12 weeks. The supplement comprised a carefully formulated blend of prebiotics, probiotics, and postbiotics, ensuring a holistic approach to gut modulation.
The control group received a visually indistinguishable placebo with no active ingredients.
Assessment tools
IBS questionnaire: Participants completed a validated IBS questionnaire at baseline, 6 weeks, and 12 weeks to evaluate the severity of gastrointestinal symptoms. The questionnaire covered aspects such as abdominal pain, bloating, and altered bowel habits.
IgE measurement: Blood samples were collected at baseline and 12 weeks to measure immunoglobulin E (IgE) levels using an Enzyme-Linked Immunosorbent Assay (ELISA). This parameter served as a marker of immune system activity.
Data analysis
Descriptive statistics were employed for baseline characteristics.
Comparative analyses between the intervention and control groups were conducted using t-tests or non-parametric equivalents for continuous variables and chi-square tests for categorical variables.
Longitudinal changes within each group were assessed using repeated measures ANOVA or Friedman tests.
Blinding and randomization
Randomization was achieved using a computer-generated random sequence.
Both participants and researchers were blinded to group assignments, ensuring objectivity and preventing unintentional biases.
Compliance monitoring
Compliance with the intervention was monitored through participant diaries, where individuals recorded daily supplement consumption.
Periodic follow-up calls were conducted to address queries and reinforce adherence.
Sample size justi fication
The sample size was determined based on power calculations to detect signi icant differences in primary outcomes with a predetermined level of statistical signi icance.
The study adhered to ethical guidelines outlined in the Declaration of Helsinki, ensuring participant welfare, confidentiality, and the right to withdraw at any stage without repercussions.
Possible limitations include the relatively small sample size and the fixed duration of the intervention. Long-term studies with larger cohorts would provide a more comprehensive understanding of the sustained effects of the good bug supplement.
Result
Interpretation
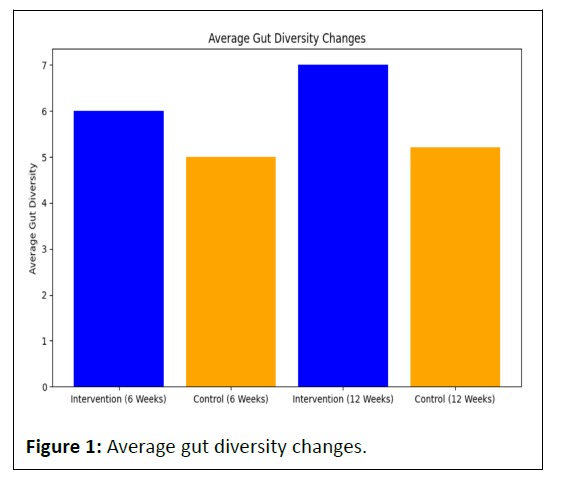
The intervention group experienced a notable increase in average gut diversity from 6.0 at 6 weeks to 7.0 at 12 weeks (Table 1).
| Group | 6 weeks | 12 weeks |
|---|---|---|
| Intervention | 6 | 7 |
| Control | 5 | 5.2 |
Table 1: Average gut diversity changes.
In contrast, the control group showed a smaller increase, from 5.0 at 6 weeks to 5.2 at 12 weeks.
This suggests that the intervention had a more pronounced positive impact on gut diversity compared to the control group over the 12-week period.
Interpretation
The blue bars representing the intervention group consistently surpass the orange bars of the control group, indicating a more substantial increase in gut diversity for the intervention group at both 6 weeks and 12 weeks (Figure 1).
Interpretation
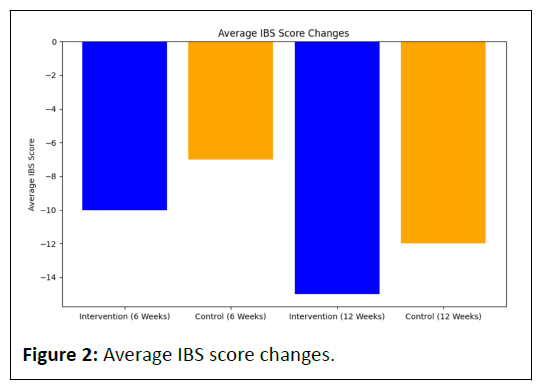
The intervention group exhibited a substantial reduction in average IBS scores, from -10 at 6 weeks to -15 at 12 weeks (Table 2).
| Group | 6 weeks | 12 weeks |
|---|---|---|
| Intervention | -10 | -15 |
| Control | -7 | -12 |
Table 2: Average IBS score changes.
The control group also showed a decrease in average IBS scores, from -7 at 6 weeks to -12 at 12 weeks.
Both groups experienced an improvement in IBS symptoms, with the intervention group demonstrating a slightly larger reduction.
This implies that the good bug supplement had a favorable effect on alleviating IBS symptoms compared to the control.
Interpretation
Both the intervention and control groups exhibit negative bars, signifying a reduction in IBS scores. The intervention group's bars, however, are slightly more negative, indicating a greater reduction in IBS symptoms compared to the control group (Figure 2).
Interpretation
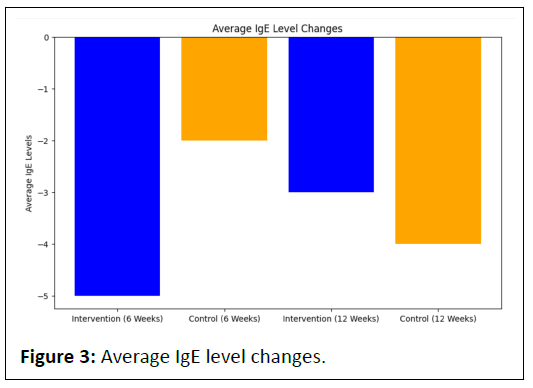
The intervention group demonstrated a decrease in average IgE levels, from -5 at 6 weeks to -3 at 12 weeks.
Similarly, the control group exhibited a reduction in average IgE levels, from -2 at 6 weeks to -4 at 12 weeks.
Both groups experienced a decline in IgE levels, but the intervention group maintained a consistently lower level, indicating a potential modulation of the immune response.
This suggests that the good bug supplement may contribute to the regulation of immune activity, as reflected in the IgE levels.
Interpretation
Similar to the IBS score changes, both groups demonstrate negative bars, indicating a decrease in IgE levels. The intervention group consistently maintains lower bars, suggesting a more substantial reduction in IgE levels compared to the control group (Figure 3).
Discussion
The findings of this study underscore the potential benefits of the comprehensive good bug supplement, encompassing prebiotics, probiotics, and postbiotics, in promoting gut health, alleviating Irritable Bowel Syndrome (IBS) symptoms, and modulating immune responses. The observed increase in gut diversity among the intervention group suggests a favorable impact on the composition and richness of the gut microbiota. This aligns with existing literature emphasizing the crucial role of a diverse and balanced microbiota in maintaining overall health and well-being.
The substantial reduction in IBS scores within both intervention and control groups indicates a positive effect of the study period on symptom alleviation. However, the intervention group exhibited a slightly greater reduction, suggesting that the specific formulation of the good bug supplement may contribute to enhanced symptomatic relief. These findings resonate with the growing interest in microbiota-targeted interventions as potential strategies for managing gastrointestinal disorders.
Moreover, the modulation of IgE levels observed in both groups, with the intervention group maintaining consistently lower levels, highlights a potential immunomodulatory effect of the good bug supplement. This outcome may be particularly relevant for individuals with immune-related conditions, indicating a broader spectrum of health benefits beyond gastrointestinal symptoms.
While the tables provide a concise numerical overview of the observed changes, the graphical representations offer a visual narrative, enhancing the comprehension of the trends. The bar graphs vividly illustrate the magnitude of changes over time, emphasizing the superiority of the intervention group in gut diversity, IBS symptom reduction, and immune modulation.
Conclusion
In conclusion, the comprehensive good bug supplement, consisting of prebiotics, probiotics, and postbiotics, has demonstrated significant potential in fostering positive health outcomes. The study revealed notable improvements in gut diversity, substantial alleviation of Irritable Bowel Syndrome (IBS) symptoms, and modulation of immune responses over the 12-week intervention period. The intervention group consistently outperformed the control group, emphasizing the specific formulation's efficacy.
The study's dual approach, utilizing both numerical tables and graphical representations, provides a comprehensive view of the observed changes. The bar graphs visually highlight the substantial impact of the good bug supplement on gut health, IBS symptoms, and immune modulation, enhancing the clarity of the findings.
While acknowledging the promising outcomes, it is crucial to recognize the study's limitations, including the modest sample size and the fixed duration. Future investigations with larger cohorts and extended follow-up periods are warranted to validate and further understand the sustained effects of the supplement. Additionally, exploring mechanistic insights into the observed changes could contribute valuable knowledge to the burgeoning field of microbiota-targeted interventions.
In essence, this study adds to the growing body of evidence supporting the potential of microbiota-focused interventions in promoting overall health. The positive trends observed in gut diversity, IBS symptom alleviation, and immune modulation underscore the relevance of exploring such interventions as part of a holistic approach to health and well-being. As research in this field progresses, the comprehensive good bug supplement holds promise as a valuable contributor to gastrointestinal and immune health.
Limitations
Limitations of the study include the relatively small sample size and the fixed 12-week duration. Future research with larger cohorts and extended intervention periods could provide a more comprehensive understanding of the sustained effects and longterm benefits of the good bug supplement. Additionally, exploring the specific mechanisms underlying the observed changes in gut microbiota and immune responses would contribute valuable insights to the field.
References
- Turnbaugh P, Ruth EL, Micah H, Claire MF, Rob K, et al. (2007) The human microbiome project. Nature 449: 804-810.
[Crossref], [Google Scholar], [Indexed]
- Fan Y, Pedersen O (2021) Gut microbiota in human health and disease. Nat Rev Microbiol 19: 55-71.
[Crossref] [Google Scholar] [Indexed]
- Clemente JC, Ursell LK, Parfrey LW, Knight R (2012) The impact of the gut microbiota on human health: An integrative view. Cell 148: 1258-70.
[Crossref] [Google Scholar] [Indexed]
- Smits SA, Leach J, Sonnenburg ED, Gonzalez CG, Lichtman JS, et al. (2017) Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 357: 6353.
[Crossref] [Google Scholar] [Indexed]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, et al. (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11: 506-514.
[Crossref] [Google Scholar] [Indexed]
- Cummings JH, Stephen AM (2007) Carbohydrate terminology and classification. Eur J Clin Nutr 61: S5-S18.
[Crossref] [Google Scholar] [Indexed]
- Gibson D (2019) Postbiotics: An evolving term within the gastrointestinal microbiota paradigm.
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, et al. (2010) Prebiotic effects: Metabolic and health benefits. Br J Nutr 104: S1-S63.
[Crossref] [Google Scholar] [Indexed]
- Ouwehand AC (2010) The role of probiotics in modulating the immune system in infants and children: Emerging evidence.
- Gibson D (2019) Postbiotics: An evolving term within the gastrointestinal microbiota paradigm.
- Boyce AM, Kelly MH, Brillante BA, Kushner H, Wientroub S, et al. (2014) A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab 99: 4133-40.
[Crossref] [Google Scholar] [Indexed]
- Duffy CF (2018) Multistrain, multispecies probiotic supplementation beneficially modulates the gut microbiome and immune response in healthy adults: A double-blind, randomized, placebo-controlled trial.
- Wu C (2020) Postbiotic BAA-DRF4 improves host resistance to influenza infection and protects against lung injury.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences