Observational Study Evaluating the Efficacy and Safety of Cartylis⢠Food Supplement in Patients Suffering from Chronical Joint Pain
Eduard Vidovic*, Florent Rigaudier and Christine Juhel
Department of Medical, University of Geneva, Geneva, Switzerland
- *Corresponding Author:
- Eduard Vidovic
Department of Medical
University of Geneva
Geneva
Switzerland
E-mail: e.vidovic@aptissen.com
Received date: August 31, 2022, Manuscript No. IPCTN-22-14430; Editor assigned date: September 05, 2022, PreQC No. IPCTN-22-14430 (PQ); Reviewed date: September 20, 2022, QC No. IPCTN-22-14430; Revised date: December 23, 2022, Manuscript No. IPCTN-22-14430 (R); Published date: January 09, 2023, DOI: 10.36648/IPCTN.8.1.002
Citation: Vidovic E, Rigaudier F, Juhel C (2023) Observational Study Evaluating the Efficacy and Safety of Cartylis™ Food Supplement in Patients Suffering from Chronical Joint Pain. J Nutraceuticals Food Sci Vol:8 No:1
Abstract
Background: Aim of this study was to analyses the clinical outcomes after 3 months of daily intake of hydrolyzed collagen containing vitamins C, D and E and selenium on joint symptoms in patients suffering from symptomatic chronical osteoarthritis.
Methods: 143 adult patients having suffered from Osteoarthritis (OA) induced joint pain for at least 3 consecutive months were included by practicing rheumatologists. The intensity of the pain at rest and during movement as well as the degree of stiffness were evaluated with Visual Analogue Scales (VAS) from 0 (no pain/stiffness) to 100 (the worst imaginable pain/stiffness), upon inclusion and after 4 (W4) and 12 (W12) weeks of Cartylis supplementation with one vial per day.
Results: After 3 months of daily intake of studied food supplement, the pain intensity both at rest and on movement as well as the stiffness were significantly reduced (p<0.001) with even more marked reduction in the subgroup of patients with higher baseline pain score.
Conclusions: Nutritional supplementation alternatives based on hydrolyzed collagen can contribute to relieving OA symptoms and reducing long-term pharmacotherapy and its potential side effects.
Keywords
Osteoarthritis; Food supplement; Hydrolyzed collagen; Selenium; Total Population (TP)
Introduction
Osteoarthritis (OA) is the most common forms of arthritis in the general population, accounting for more pain and functional disability than any other musculoskeletal disease. Is the result of mechanical and biological phenomena that destabilise the balance between cartilage regeneration and deterioration? OA causes joint pain and stiffness, inflammation, or even joint effusion there are currently no approved disease modifying drugs for OA. Its management is primarily targeting analgesic and anti-inflammatory effects. In the absence of effective pharmacotherapy, many patients with OA turn to nutritional supplements and nutraceuticals, including collagen derivatives [1]. Nutraceuticals are orally administrated, biologically active compounds that have been shown to slow down the progression of the signs of aging. Hydrolyzed collagen, as a nutraceutical supplement, has been extensively shown to benefit human cartilage, skin and connective tissues [2]. Collagen hydrolysates are terms used to describe collagens that have been broken down into small peptides and amino acids in the presence of collagenases and high pressure. Several studies described the mechanism of absorption and distribution of collagen peptides in the body. It has been demonstrated that C14 labeled collagen peptides can reach skin, cartilage, bones, and muscles and remain in these tissues up to 14 days after a single ingestion. Thus, nutritional alternatives can contribute to relieving OA symptoms and reducing long term medicine consumption and its potential side effects. Cartylis is a food supplement based on hydrolysed collagen. It also contains vitamins C, D and E, and selenium. These different ingredients have synergistic properties that are thought to favour cartilage maintenance and reduce inflammation [3]. The aim of this observational study is to document in real life the clinical outcomes after 3 months of daily intake of cartylis on joint symptoms in patients suffering from symptomatic chronical osteoarthritis.
Materials and Methods
Adult patients having suffered from joint pain for at least 3 consecutive months, of which at least one joint had not been treated by infiltration or viscos supplementation within the previous 2 months, having no ongoing anti-inflammatory treatment and taking no other food supplements, were included by rheumatologists in their daily practice [4]. The intensity of the pain at rest and during movement as well as the degree of stiffness were evaluated with Visual Analogue Scales (VAS) from 0 (no pain/stiffness) to 100 (the worst imaginable pain/stiffness), upon inclusion and after 4 (W4) and 12 (W12) weeks of Cartylis supplementation with one vial per day. These evaluations were conducted for the Total Population (TP) and a Sub Population (SP) of patients with more intense symptoms, objectivized by a baseline VAS ≥ 30 (SP).
In total, 143 patients were included, of which 116 qualified to be evaluated for efficacy. The average age was 64.7 ± 9.3 years old, and 79% were women. The joint pain, present in the last 7 years on average and triggered most frequently by an arthritis flare up, had been mostly treated with analgesics (70%), infiltrations (48%), or NSAIDs (46%), and more rarely with phytotherapy or a food supplement (13%) (Table 1).
The Patient Global Impression of Improvement (PGII) and the overall satisfaction regarding the studied food supplement (Likert 5 point scale) were evaluated at 4 and 12 weeks. Finally, the consumption of analgesics and anti-inflammatory drugs and the undesirable side effects were noted [5]. The tolerability was evaluated by the description and the frequency of all events. The AE’s were coded according to the MedDRA classification.
| Previous treatments | Non (N/%) | Oui (N/%) | Total (N/%) | |||
|---|---|---|---|---|---|---|
| Analgesics intermittent | 32 | 29.9 | 75 | 70.1 | 107 | 100 |
| Oral NSAID intermittent | 89 | 83.2 | 18 | 16.8 | 107 | 100 |
| Analgesics | 88 | 82.2 | 19 | 17.8 | 107 | 100 |
| Weak opioides | 105 | 98.1 | 2 | 1.9 | 107 | 100 |
| Oral NSAID regularely | 64 | 59.8 | 43 | 40.2 | 107 | 100 |
| Topical NSAID | 89 | 83.2 | 18 | 16.8 | 107 | 100 |
| Systemic slow acting drugs for OA | 88 | 82.2 | 19 | 17.8 | 107 | 100 |
| Phytotherapy or food supplements | 93 | 86.9 | 14 | 13.1 | 107 | 100 |
| IA corticosteroid injections | 68 | 63.6 | 39 | 36.4 | 107 | 100 |
| IA hyaluronic acid injections | 81 | 75.7 | 26 | 24.3 | 107 | 100 |
Table 1: Previous non-surgical treatments N=107.
Statistics
The characteristics of the subjects at inclusion in the study were described by means and standard deviation for the quantitative variables and by frequencies and numbers for the qualitative variables.
The primary endpoint is defined as the variation in pain or discomfort intensity on a Visual Analog Scale (VAS) of the most painful joint at baseline. It was compared between inclusions and after 4 weeks and 12 weeks of supplementation using paired Student tests. These analyses were performed for the intensity of pain (or discomfort) at rest and during movement [6].
Secondary endpoints: Joint stiffness was compared between inclusions and after 4 weeks and 12 weeks of supplementation using paired student tests. These changes were compared between D0 and W4, D0 and W12 and between W4 and W12. Satisfaction assessed on a Likert scale and perceived global improvement assessed by the PGII were analyzed and compared between the 4 weeks and 12 weeks follow up visits using paired Wilcoxon signed rank tests.
Consumption of analgesics and anti-inflammatory drugs was quantified and described at each visit. The comparison between the 4 weeks and 12 weeks follow up visits was made using McNemar's chi-square tests. The number of days of taking at least one analgesic/NSAID was not compared between visits.
Results
The most painful joint, most frequently situated in the lower (46%) or upper (36%) limbs, presented moderate pain (VAS=36.2 ± 25.5) at rest and more severe pain during movement (VAS=59.1 ± 20.2) [7]. Stiffness was also high (VAS=54.4 ± 23.3) as shown on Tables 2 and 3.
| Localisation of the most painful joint | N | % |
|---|---|---|
| Head | 1 | 1 |
| Cervical spine | 4 | 4 |
| Shoulder | 3 | 3 |
| Lumbar spine | 2 | 2 |
| Pelvis | 8 | 7.9 |
| Upper limb | 36 | 35.6 |
| Lower limb | 47 | 46.5 |
| Total | 101 | 100 |
Table 2: Localization of the most painful joint N=101.
| Pain at baseline | N | DM | Mean | SD | Median | Minimum | Maximum | Lower threshold CI95 | Upper threshold CI95 |
|---|---|---|---|---|---|---|---|---|---|
| Pain at rest | 116 | 0 | 36.2 | 25.5 | 37 | 0 | 100 | 31.5 | 40.9 |
| Pain at movement | 116 | 0 | 59.1 | 20.2 | 60 | 17 | 100 | 55.4 | 62.8 |
| Stiffness | 116 | 0 | 54.4 | 23.3 | 54.5 | 0 | 100 | 50.1 | 58.7 |
Table 3: Joint symptoms (pain and stiffness) at baseline N=116.
With a daily dose of the food supplement, the intensity of the pain at rest was progressively reduced by up to 37% (median) at W12, where the value of the VAS was significantly lower than that of the base VAS (25.9 ± 24.1, p<0.01). The analysis by sub-group showed a higher and more significant pain reduction as of W4 (median: -17%), with maximum reduction at W12 (median: -55%) for SP patients.
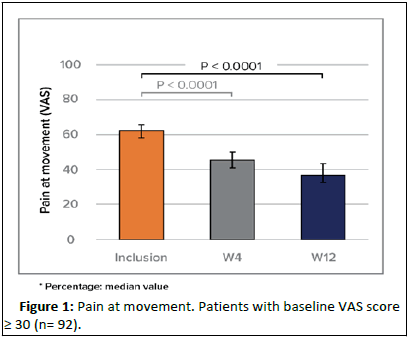
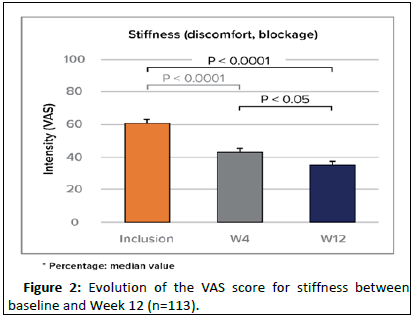
The pain relief was more noticeable during movement as of W4 (-25%) and remained significant at W12 (median: -36%). After 4 and 12 weeks, the stiffness was significantly reduced by 23% and 39% (median). The improvement was slightly more noticeable for SP patients (Figure 1).
After 3 months of supplementation, 77% of patients felt there was a significant improvement and 62% declared to be satisfied with the food supplement. Between the inclusion and W4 and between W4 and W12, 60% and 68% of patients respectively had consumed neither analgesics nor anti-inflammatory drugs. Among the consumers, the number of days per month with a consumption of medicines tended to lower (median of 5 at W4 against 4 at W12). The treatment was globally very well tolerated. 9 study participants (6.2%) have reported some transitory intestinal disorders but the causality and relatedness to Cartylis could not have been clearly established (Figure 2).
Discussion
Collagen hydrolysates have demonstrated some evidence of efficacy in a handful of controlled clinical trials, but their ability to treat and reverse advanced joint disease remains to be confirmed in larger clinical trials, as is the case for other nutritional supplements. Here the authors acknowledge the limitations of this open label PMS study such as the absence of placebo or active control group as well as the absence of more objective parameters. Still, pain, function and stiffness as reported by patients on a VAS remain crucial clinical endpoints, especially for a chronical disabling disease like OA. We therefore consider that these results add to a more comprehensive outlook on the efficacy and safety of collagen derivatives in OA symptom relief, hence their place as an alternative to long term pharmacotherapy.
Conclusion
This real life study of patients suffering from osteoarthritis joint pain, functional disability and stiffness demonstrated the benefit of the cartylis food supplement in the conservative, non-pharmacological treatment of joint pain and functional discomfort, notably for patients with moderate to severe symptoms.
Its excellent tolerability and its favourable impact on analgesic and anti-inflammatory drug consumption confirm the benefit of using cartylis to relieve chronic OA symptoms.
References
- Mobasheri A, Mahmoudian A, Kalvaityte U, Uzieliene I, Larder CE, et al. (2021) A white paper on collagen hydrolyzates and ultrahydrolyzates: potential supplements to support joint health in osteoarthritis? Curr Rheumatol Rep 23:78
[Crossref] [Google Scholar] [PubMed]
- Sato Y, Mera H, Takahashi D, Majima T, Iwasaki N, et al. (2017) Synergistic effect of ascorbic acid and collagen addition on the increase in type 2 collagen accumulation in cartilage like MSC sheet. Cytotechnology 69: 405–416
[Crossref] [Google Scholar] [PubMed]
- Muchova K, Hearnden V, Michlovska L, Vistejnova L, Zavadakova A, et al. (2021) Mutual influence of selenium nanoparticles and FGF2-STAB® on biocompatible properties of collagen/chitosan 3D scaffolds: In vitro and ex vivo evaluation. J Nanobiotechnol 19:103
[Crossref] [Google Scholar] [PubMed]
- Germain H, Lengele L, Charles A, Reginster JY, Bruyere O (2020) Role of collagen derivatives in osteoarthritis and cartilage repair: A systematic scoping review with evidence mapping. Rheumatol Ther 7:703–740
[Crossref] [Google Scholar] [PubMed]
- McAlindon TE, Nuite M, Krishnan N, Ruthazer R, Price LL, et al. (2011) Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarthr Cartil 19:399–405
[Crossref] [Google Scholar] [PubMed]
- Benito-Ruiz P, Camacho-Zambrano MM, Carrillo-Arcentales JN, Mestanza-Peralta MA, Vallejo-Flores CA, et al. (2009) A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int J Food Sci Nutr 2:99–113
[Crossref] [Google Scholar] [PubMed]
- Bobacz K, Erlacher L, Smolen J, Soleiman A, Graninger WB (2004) Chondrocyte number and proteoglycan synthesis in the aging and osteoarthritic human articular cartilage. Ann Rheum Dis 63:1618–622
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences