Nootropic and Anti-anxiety Effects of Olive Oil: Relationship with Dopamine and Serotonin Metabolism
M. Atif Raza Cheema, Khalid Mahmood and Darakhshan J Haleem
Atif Raza Cheema M1,2*, Khalid Mahmood2 and Darakhshan J Haleem2
1Faculty of Pharmacy and Pharmaceutical Sciences, Department of Pharmacology, University of Karachi, Karachi, Pakistan
2Neuroscience Research Laboratory, Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan
- *Corresponding Author:
- Atif Raza Cheema M
Neuroscience Research Laboratory (P-102)
Dr. Panjwani Center for Molecular Medicine
and Drug Research (PCMD), International
Center for Chemical and Biological Sciences (ICCBS)
University of Karachi, Karachi-75270, Pakistan
Tel: 922199261300-07
E-mail: atifpharmacist3@iccs.edu
Received Date: January 24, 2018 Accepted Date: April 18, 2018 Published Date: April 26, 2018
Citation: Cheema MAR, Mahmood K, Haleem DJ (2018) Nootropic and Anti-anxiety Effects of Olive Oil: Relationship with Dopamine and Serotonin Metabolism. J Nutraceuticals Food Sci Vol.3:No.1:4.
Abstract
In recent years, interest in the use of nutraceuticals has risen substantially. Olive oil has been shown to produce a number of therapeutically important effects due to its antioxidant property. The present study concerns neurochemical and behavioral effects of long term administration of low and high doses (0.1 mL/kg and 0.25 mL/kg) of olive oil and associated antioxidant effects in rats. Long term administration of low dose of olive oil increased motor activity in an open field, decreased anxiety in elevated plus maze test, and enhanced memory in Morris water maze test. Whole brain levels of serotonin increased with low dose of olive oil while homovanillic acid (HVA), a metabolite of dopamine increased with both doses of olive oil. Low dose of olive oil increased glutathione peroxidase activity whereas high dose of olive oil decreased malondialdehyde levels in plasma. The results show that particularly low doses of olive oil reduce anxiety and improve learning and memory together with antioxidant properties, brain dopamine and serotonin also play important role in the therapeutically important effects of olive oil.
Keywords
Olive oil; Anxiety; Memory; Dopamine; Serotonin
Introduction
In recent years, interest in the use of nutraceuticals has risen substantially, largely because of their efficacy, fewer side effects, and cost efficiency. There is growing need of nootropic agents because the currently available cognitive-enhancing drugs (psychostimulants) have unwanted side effects such as psychotic symptoms and abuse potential [1]. Remission rate and treatments for psychiatric illnesses such as depression and anxiety are also not satisfactory [2].
The fruit of Olea europaea L. (Family: Oleaceae) is commonly known as olive. It is a major component of the Mediterranean diet. In the last few decades, global consumption of olive oil has increased due to increased awareness of its health benefits [3,4]. Olive oil has been used in traditional medicine due to its antihypertensive and cardioprotective effects. It also has anti-inflammatory, analgesic, and anticancer effects [5]. However, effects of this oil on monoamine metabolism have not been widely investigated [6].
Despite a number of beneficial effects only few studies have been performed on the effects of olive oil on anxiety and cognition [6,7]. It has also been reported that 4 weeks administration of olive oil at dose of 0.25 mL/kg produces antidepressant and antianxiety effects and this was associated with a decrease in brain serotonin [5-hydroxytryptamine (5- HT)] and dopamine (DA) metabolism [6]. In the present study, potential antianxiety effects of low and high doses of olive oil were determined after one and five weeks of administration. The anti-anxiety effect was produced after 5 weeks but not one week of oil administration. Animals were later tested on water maze for learning acquisition and memory retention. The animals were killed to collect brain and plasma samples for determining 5-HT and DA metabolism in the whole brain and malondialdehyde (MDA) and glutathione peroxidase (GSHPX) activity in the plasma. Food intake and change in body weight during 5 weeks treatment were also monitored.
Materials and Methods
Experimental animals
Locally bred male albino Wistar rats, weighing 180-230 g (age approximately 7 weeks) were obtained from Animal Resource Facility of Dr. Panjwani Center for Molecular Medicine and Drug Research. They were individually housed in opaque cages (to avoid effect of social interaction) at controlled room temperature (22 ± 2°C) and humidity (55 ± 10%) under 12-h light/dark cycle (lights on at 7:00 h). They were provided standard rodent diet [8] and water ad libitum during the entire study period. This study was approved by Institutional Animal Care and Use Committee, International Center for Chemical and Biological Sciences (ICCBS), University of Karachi (Protocol#: 2014-0016). Animal study protocol was conducted in accordance with National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Oil, chemicals and reagents
Olive oil (100% pure) was purchased from a retail store of Karachi (imported from Sasso® Via 31 Tavarnelle Val di Pesa, Firenze, Italy).
2-Thiobarbituric acid, 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) or Ellman’s reagent, disodium hydrogen phosphate anhydrous (di-basic) (Na2HPO4), Ethylene-diaminetetra-acetic acid disodium salt 2-hydrate (EDTA-Na2), glutathione (GSH), hydrogen peroxide solution 35% by weight (H2O2), sodium azide, sodium carbonate, sodium dihydrogen phosphate dihydrate (monobasic) (NaH2PO4.2H2O), and trichloroacetic acid (TCA), DA, homovanillic acid (HVA), dihydroxyphenyl acetic acid (DOPAC), 5-HT creatinine sulfate, 5-hydroxyindole acetic acid (5-HIAA), and sodium octyl sulfate (SOS) were procured from Sigma (St. Louis, MO, USA). HPLC grade chemicals and reagents were used in HPLC-ECD (High Performance Liquid Chromatography with Electrochemical Detector), whereas all other chemicals and reagents were of analytical grade.
Experimental protocol
Twenty-one rats were divided in to three groups (n=7). All treatments were orally administered at 09:30 to 10:00 h daily for five weeks. Group 1 (control) received tap water. Groups 2 (olive oil 10) and 3 (olive oil 25) were treated with 0.1 mL/kg and 0.25 mL/kg of olive oil, respectively. Animals were acclimatized for one week before the start of experiment. Cumulative food intake and change in body weight gain were monitored during the five weeks treatment. Activity in a home cage and open field was monitored after five weeks of drug treatment from 10:30 to 11:00 h and 11:30 to 12:00 h, respectively.
Twenty-one rats were divided in to three groups (n=7). All treatments were orally administered at 09:30 to 10:00 h daily for five weeks. Group 1 (control) received tap water. Groups 2 (olive oil 10) and 3 (olive oil 25) were treated with 0.1 mL/kg and 0.25 mL/kg of olive oil, respectively. Animals were acclimatized for one week before the start of experiment. Cumulative food intake and change in body weight gain were monitored during the five weeks treatment. Activity in a home cage and open field was monitored after five weeks of drug treatment from 10:30 to 11:00 h and 11:30 to 12:00 h, respectively.
After centrifugation at 4,000 rpm for 12 min the plasma was separated [9]. Brain and plasma samples were kept at -80°C for neurochemical analysis by HPLC-ECD and analysis of antioxidant enzymes using microplate absorbance reader, respectively.
Food intake and body weights
Cumulative food intake (g) was determined weekly between 08:30 and 09:30 h by taking the difference of food given on week 1 and food left on the following week. Percentage change in body weight was calculated weekly as: (current body weight/ preceding week body weight) × 100 [10].
Behavioral studies
Home cage activity test: Specially designed transparent Perspex cages (26 × 26 × 26 cm) with sawdust-covered floor were used to monitor activity in the familiar environment. Half an hour after administration of water or oil, rats were placed in separate activity cages to get familiar with the environment. Numbers of cage crossings were monitored for 10 min. Home cage activity was monitored in a balanced design [11].
Open field test: Open field activity test is used to assess behavioral responses such as locomotor activity and exploration in a novel environment. The apparatus consisted of a square area (76 × 76 cm) with opaque walls 42 cm high. The floor was divided into 25 equal squares (15 cm). One and a half hour after the administration of water or oil, a single animal was taken out from its home cage and placed in the central square of the open field. Latency period (s) to move from the central square and number of squares crossed with all four paws were recorded for a period of 5 min as reported earlier [11,12]. Activities of control and test groups were monitroed in a balanced design to avoid order and time effect.
Elevated plus maze test: Elevated plus maze (EPM) model was used to monitor animal’s natural behavior which is fear of novel and open areas. Rats normally spend more time exploring the enclosed elevated arm of a maze compared with the open and exposed elevated arm. The anxiolytic effects of antianxiety drugs have been determined by these typical patterns of behavior. Rats treated with antianxiety drugs generally spend more time in open elevated arms compared to controls. Plus maze apparatus is used to assess the anxiogenic or anxiolytic activity of novel drugs. The maze consists of four arms of equal size arranged in shape of ‘plus’ sign having 50 cm length and 10 cm width and elevated (at a height of 60 cm) from the floor. Two opposite arms of the maze were open while other two were closed joined together by the central area of 10 × 10 cm. Closed arm contains 15 cm high side and end walls. To monitor anxiolytic effect, three hour after the administration of water or oil a single rat was placed in the central area of the apparatus facing the corner between a closed and an open arm. The time spent in open arm by the animal was recorded for 5 min. Both water- and oil-treated groups were tested in a balanced design to avoid order effect [13].
Morris water maze test: Morris water maze test was performed to study learning acquisition and memory retention in rats as developed earlier [14]. The apparatus used for water maze test was a white plastic circular tank with a diameter of 90 cm and height of 60 cm. Opaque milky water (24 ± 2°C) was added in the pool to a depth of 30 cm. A square platform (10 × 10 cm2) was submerged 2 cm below the water surface and positioned in a fixed location (North quardrant) to provide an escape from water for rats [15]. Water maze tank was placed in a room surrounded by invariable visual cues (window, cabinets, equipments, etc.) Which remained invariable with the water maze during the entire experiment. Water maze was arbitrarily divided into four equal quadrants (North, South, East, and West). This test was performed after five weeks of daily administration of olive oil.
In the training phase, each animal was allowed in a specific time to locate the hidden platform in three trials from three different start position, one from each quadrant (other than the quadrant with hidden platform). During each trial, an animal was placed in the water maze, facing the tank wall and given 120 s to locate and climb onto the platform. The rat was allowed to stay on the platform for 10 second. If it failed to locate the platform within the allowed time it was guided gently onto the hidden platform. After each trial, animal was placed in a cage containing dry towel for 60 s before the next trial.
In the test session, rats were tested for learning acquisition as well as memory retention by placing the rat in the South quadrant and monitoring time to reach the platform. Rats were tested for learning acquisition 2 hours after the trial phase between 12:30 and 13:30 h while memory retention was monitored next day between 9:30 and 10:30 h.
Analysis of antioxidant enzymes
Determination of MDA content: The procedure for estimation of lipid peroxidation was performed as described previously [16] with slight modifications. An aliquot of 100 μL plasma and 2 mL of 0.375% TBA in 15% TCA were thoroughly mixed in test tubes. This mixture was placed in boiling water for 20 min and allowed to cool in ice-cold water at 4°C. After centrifugation at 2,000 × g for 10 min (4°C), the resulting clear supernatant of light pink color (250 μL) was collected and transferred to 96-well microplate. The absorbance of the supernatant was recorded at 532 nm in microplate absorbance reader (SunriseTM, Tecan Trading AG, Switzerland). The amount of TBA reactants was calculated using molar extinction coefficient of malondialdehyde (1.56 × 105). The data was expressed as μmoles of MDA per liter of rat plasma.
Determination of GSH-Px activity: GSH-Px activity of plasma was measured by the method of [17,18]. A mixture containing 30 μL of sodium phosphate buffer (0.1 M, pH 7.4), 20 μL of glutathione (2 mM), 30 μL of plasma, 10 μL of sodium azide (10 mM), and 10 μL of hydrogen peroxide solution (1 mM) in a 2 mL microcentrifuge tube was incubated for 15 min at 37°C. The reaction was stopped by vigorously injecting a total of 50 μL 5% TCA. After centrifugation at 8,325 rpm for 5 min (4°C), the resulting supernatant (25 μL) was collected and transferred to 96-well microplate. 50 μL of sodium phosphate buffer (0.1 M, pH 7.4) and 175 μL of DTNB (1 mM) were added to supernatant. The mixture was given a shake duration of 10 s by placing the 96-well microplate in the absorbance reader.
The absorbance was measured at 420 nm in microplate absorbance reader (SunriseTM, Tecan Trading AG, Switzerland). An appropriate control was run along each plasma sample and its reaction was immediately stopped at 0 min. GSH-Px activity was expressed as the μmoles of GSH converted to GSSG per min per milliliter of rat plasma.
Neurochemical analysis of biogenic amines and metabolites
Biogenic amines and metabolites were extracted as reported earlier 19. 5 times volume of the extraction medium (containing sodium metabisulfite (0.1%), perchloric acid (0.4 M), EDTA (0.01%), cysteine (0.01%) was added to the brain tissue. Brain sample was homogenized (by using Ultra-Turrax T8 homogenizer, IKA®-Werke, Germany) and centrifuged twice at 12,000 rpm for 5 minutes at 4°C. Supernatants were collected and injected (20 μL) to HPLC-ECD for simultaneous measurements of DA, and 5-HT as well as their metabolites, DOPAC, HVA, and 5-HIAA as previously reported [19,20]. A 5 μm ODS separation column (0.6 × 250 mm) was used. 0.1 M sodium phosphate buffer (pH 2.9) containing methanol (10%), EDTA (0.005%), and sodium octyl sulfate (0.023%) was used as a mobile phase at an operating pressure of 2000-3000 psi on HPLC pump (Shimadzu, Kyoto, Japan).
Electrochemical detection was done at an operating potential of +0.8 to +1.0 V by using Schimadzu L-ECD-6A detector. The individual components of sample as well as standard were identified and quantified by comparing their retention times and area under the peaks by using a software (Shimadzu’s LC solution).
Statistical Analysis
Food intake, and body weight as well as home cage, open field, and elevated plus maze activities were analyzed by oneway analysis of variance (ANOVA), using IBM SPSS Statistics (version 15.0). Post hoc comparison was done by Tukey’s test and p-values less than 0.05 were taken as statistically significant.
Results
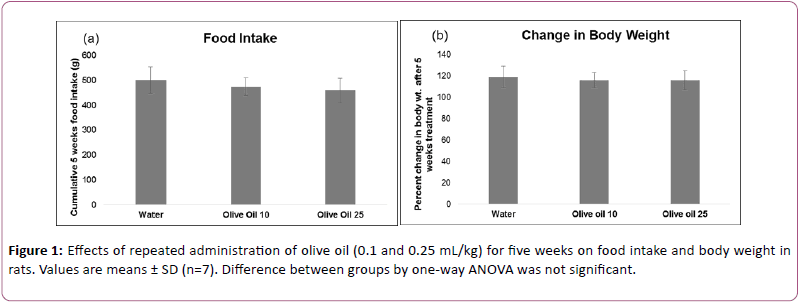
Effects of olive oil on food intake and body weights
Effects of olive oil on food intake and percent change in body weight are shown in Figure 1. Analysis of the data by one-way ANOVA showed that the effects of oil treatment on food intake (F (4,30)=2.125, p>0.05) and body weight (F (4,30)=2.157, p>0.05) were not significant.
Effects on home cage, open field, and elevated plus maze activities
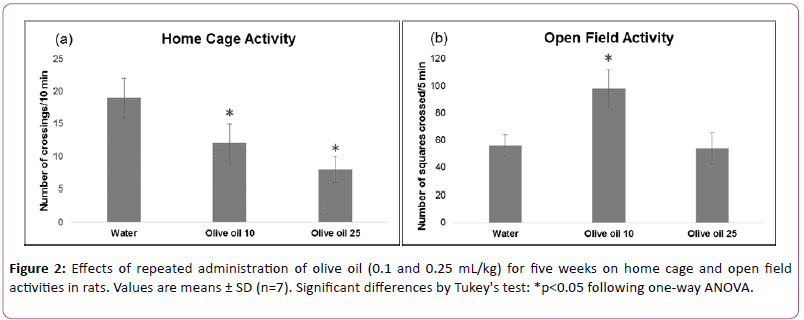
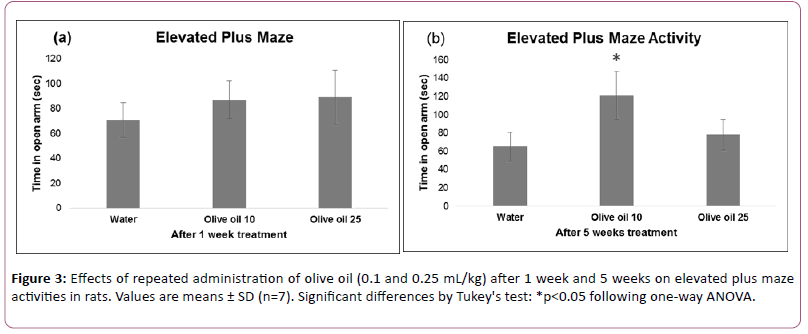
Effects of olive oil treatment on activity in home cage, open field, and elevated plus maze performance are shown in Figures 2 and 3.
Data analyzed using one-way ANOVA showed significant treatment effects on home cage (F (2,18)=35.489, p<0.01), open field (F (2,18)=24.992, p<0.01) and elevated plus maze (after 5 weeks treatment) (F(2,18)=15.064, p<0.01).
Effects on elevated plus maze (after 1week treatment) (F (2,18) =2.305, p>0.01) were not significant. The post hoc analysis by Tukey’s test showed a decrease of motor activity in the home cage.
Lower dose of olive oil increased exploratory activity in open field as well as time spent by animals in open arm of plus maze whereas higher dose of olive oil did not alter these.
These results suggest that both doses of olive oil administered for five weeks decreased motor behavior in the familiar environment of activity cage. Exploratory activity in open field is increased by low dose of olive oil. An increase in time spent in open arm showed antianxiety-like effect of low dose of olive oil after 5 weeks treatment.
Effects of olive oil on learning acquisition and memory retention
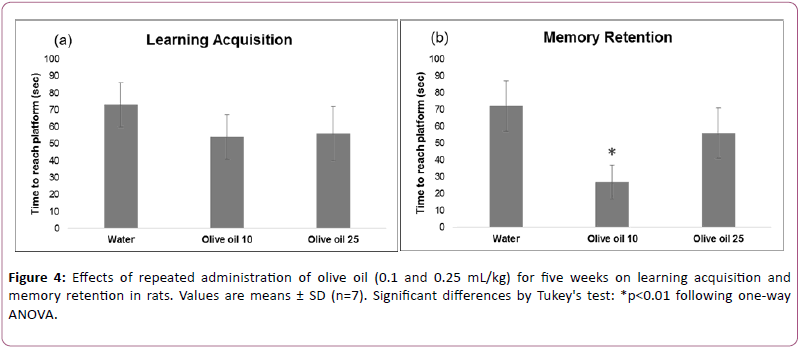
Figure 4 shows effects of the olive oil treatment on water maze performance.
Data analyzed using two-way ANOVA (repeated measure design) showed significant effects of repeated measure (F (1,18) =10.045, 18; p<0.01), treatment (F (2,18) =12.415, p<0.01), and interaction between repeated measure and treatment (F (2,18)=10.209, p<0.01).
The post hoc analysis by Tukey’s test showed that administration of lower but not higher dose of olive oil improved memory retention. Effects of both doses of olive oil on learning acquisition were not significant.
Effects of olive oil on antioxidant enzymes
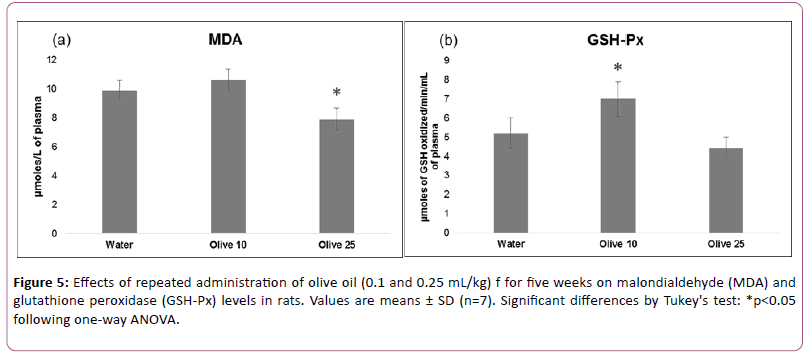
Figure 5 shows effects of olive oil treatment on plasma MDA levels and GSH-Px activity.
Data analyzed using one-way ANOVA showed significant effects on MDA (F (2,18)=24.347, p<0.01) and GSH-Px (F (2,18)=20.063, p<0.01). The post hoc analysis by Tukey’s test showed that administration of higher but not lower dose of olive oil decreased MDA by 20%. While administration of lower but not higher dose of olive oil increased GSH-Px by 35%.
These results suggest that higher dose of olive oil produces antioxidant effect by decreasing lipid peroxidation. An increase in GSH-Px enzyme activity showed antioxidant effect of lower dose of olive oil.
Effects of olive oil on biogenic amines and metabolites
Effects of olive oil treatment on biogenic amines and their metabolites are given in Table 1.
Data analyzed using one-way ANOVA showed significant effects on HVA (F (2,18) =115.271, p<0.01), 5-HT (F (2,18) =5.139, p<0.05), and DA (F (2,18)=5.892, p<0.05). Effects on DOPAC (F (2,18) =1.865, p>0.05), and 5-HIAA (F (2,18) =1.073, p>0.05) were not significant. The post hoc analysis by Tukey’s test showed that administration of both doses of olive oil increased HVA concentration. Lower but not higher dose of olive oil increased 5-HT concentration. Administration of olive oil had no effect on DA concentration.
Discussion
Effects of long term administration of olive oil on brain DA and 5-HT metabolism in relation to anxiety, memory, motor activity, and antioxidant properties were monitored in the present study. A consistent finding of this study is an increase in HVA concentration by both doses of olive oil. On the other hand, 5-HT levels increased in low dose olive oil-treated animals, but the increases were not significant at higher dose. Interestingly, enhancement in open field exploration and antianxiety-like effects in elevated plus maze were also produced at low doses only. Moreover, animals treated with low dose olive oil exhibited improved performance in water maze test. Both doses of olive oil exhibited an antioxidant effects. However, treatment of the animals with low or high doses of olive oil produced no effect on food intake and body weight. The results suggest a potential antianxiety and nootropic effect of low dose of olive oil, and a role of DA as well as 5-HT in these effects.
Other authors have reported that long term oral administration of extra-virgin olive oil (EVOO) enriched with polyphenols (total polyphenolic concentration of the original EVOO: 210 mg/kg, gallic acid equivalent) improved performance in T-maze test and object recognition task in senescence-accelerated mouse-prone 8 (SAMP8) [7]. In the present study, very low doses of pure olive oil were used. We found that oral administration of 0.1 mL/kg olive oil for 5 weeks improved performance in Morris water maze test suggesting that long term intake of olive oil can improve cognitive performance (Figure 4).
Previous studies have explained the memory-enhancing effects of enriched extra-virgin olive oil in terms of antioxidant properties of active components, including hydroxytyrosol, tyrosol, oleuropein, deacetoxy-ligstroside aglycon, and acetoxypinoresinol [7,21]. The present results of improved performance in water maze at low but not high doses of olive oil cannot be explained in terms of antioxidant effects of the oil because antioxidant effects occurred at both doses (Figure 5). An increase in DA neurotransmission is also often linked with improved performance in Morris water maze test [22,23]. However, in the present study HVA levels also increased at both doses of olive oil (Table 1). The present results on an improved cognition at low but not high doses of olive oil suggest that enhanced 5-HT neurotransmission at low doses is possibly involved in the memory-enhancing effects of olive oil. Indeed, an increase in 5-HT neurotransmission in tryptophantreated rats has been shown to improve performance in Morris water maze test [24]. In the quest of why low but not high doses are effective in improving cognition, reducing anxiety, and increase 5-HT metabolism, it is important to note that a number of active components have been isolated from olive oil and only few of these have reported to increase cognitive properties [25]. It is also possible that some components present in olive oil impair memory and are effective only at high doses. These components, if present, can also mask memory-enhancing effects of reported memoryenhancing components in the oil.
| Parameters (ng/g) |
Water | Olive 10 | Olive 25 |
|---|---|---|---|
| DA | 726 ± 77 | 755 ± 66 | 625 ± 79 |
| HVA | 70 ± 13 | 271 ± 38** | 120 ± 20** |
| DOPAC | 67 ± 10 | 71 ± 11 | 78 ± 9 |
| 5-HT | 528 ± 68 | 632 ± 54* | 571 ± 60 |
| 5-HIAA | 194 ± 45 | 221 ± 38 | 201 ± 18 |
* p<0.05, ** p<0.01 from water treated controls
Abbreviations: DA: Dopamine; DOPAC: Dihydroxyphenyl Acetic Acid; HVA: Homovanillic Acid; 5-HT: 5-hydroxytryptamine (serotonin); 5-HIAA: 5-Hydroxyindole Acetic Acid; Olive 10 (0.1 mL/kg); Olive 25 (0.25 mL/kg).
Table 1: Effects of 5 weeks administration of olive oil on biogenic amines and their metabolites.
Other authors have shown an antianxiety as well as antidepressant effect of higher dose of olive oil administered for 4 weeks, which was associated with a decrease in brain 5- HT metabolism 6. A decrease in brain 5-HT metabolism following 5 weeks administration of high dose of olive oil did not occur in the present study (Table 1). In general, the present and previous findings tend to suggest an increase or decrease in brain 5-HT metabolism following long term administration of low or high dose of olive oil. In the quest of why low but not high dose of olive oil increase brain 5-HT metabolism, it is important to note that neurochemical effects of active component olive oil have not been explored. Further studies may well explain why low but not high doses of olive oil increase 5-HT metabolism. The mechanism by which olive oil can increase brain 5-HT as well as DA metabolism also requires further investigations.
An increase in exploratory activity in open field by olive oil has been reported previously [26] and in the present study (Figure 2b). Additionally, we found a decrease in activity in the familiar environment (Figure 2a). Benzodiazepines decrease anxiety in open field. Conventional anxiolytics like diazepam increase activity in open field. Olive oil has anxiolytic-like effect similar to benzodiazepines. Benzodiazepines at low dose decrease activity in the familiar environment [27] and increase activity in novel environment. This study also showed that low but not high dose is anxiolytic.
Conclusion
In conclusion, the present results tend to suggest that together with antioxidant properties, low dose of olive oil also produces antianxiety and memory-enhancing effect. Neurochemical studies on the effects of active components in olive oil may help to explain the mechanisms involved in its antianxiety and/or nootropic effects of olive oil. At least some of its more active components seem promising candidates for treating anxiety and memory deficits.
Acknowledgements
The authors are thankful to director Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi for providing faculty research grants.
References
- Haleem DJ (2013) Extending therapeutic use of psychostimulants: Focus on serotonin-1A receptor. Prog Neuropsychopharmacol Biol Psychiatry 46: 170-180.
- Masi G, Liboni F, Brovedani P (2010) Pharmacotherapy of major depressive disorder in adolescents. Expert Opin Pharmacother 11: 375-386.
- Chih H, James AP, Jayasena V, Dhaliwal SS (2013) Effect of growing location, malaxation duration and citric acid treatment on the quality of olive oil. J Sci Food Agric 93: 1272-1277.
- Bhatnagar A, Kaczala F, Hogland W, Marques M, Paraskeva CA, et al. (2014) Valorization of solid waste products from olive oil industry as potential adsorbents for water pollution control-a review. Environ Sci Pollut Res Int 21: 268-298.
- Fezai M, Senovilla L, Jemaà M, Ben-Attia M (2013) Analgesic, anti-inflammatory and anticancer activities of extra virgin olive oil. J Lipids 2013: 1-7.
- Perveen T, Hashmi BM, Haider S, Tabassum S, Saleem S, et al. (2013) Role of monoaminergic system in the etiology of olive oil induced antidepressant and anxiolytic effects in rats. ISRN Pharmacol 2013: 1-5.
- Farr SA, Price TO, Dominguez LJ, Motisi A, Saiano F, et al. (2012) Extra virgin olive oil improves learning and memory in SAMP8 mice. J. Alzheimers Dis 28: 81-92.
- Bocarsly ME, Barson JR, Hauca JM, Hoebel BG, Leibowitz SF, et al. (2012) Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol Behav 107: 568-575.
- Todorova I, Simeonova G, Kyuchukova D, Dinev D, Gadjeva V (2005) Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp Clin Path 13: 190-194.
- Haleem DJ, Ikram H, Haider S, Parveen T, Haleem MA (2013) Enhancement and inhibition of apomorphine-induced sensitization in rats exposed to immobilization stress: Relationship with adaptation to stress. Pharmacol Biochem Behav 112: 22-28.
- Ikram H, Haleem DJ (2011) Attenuation of apomorphine-induced sensitization by buspirone. Pharmacol Biochem Behav 99: 444-450.
- Kennett GA, Dickinson SL, Curzon G (1985) Central serotonergic responses and behavioural adaptation to repeated immobilisation: The effect of the corticosterone synthesis inhibitor metyrapone. Eur J Pharmacol 119: 143-152.
- Haider S, Saleem S, Tabassum S, Khaliq S, Shamim S, et al. (2013) Alteration in plasma corticosterone levels following long term oral administration of lead produces depression like symptoms in rats. Brain Dis 28: 85-92.
- Morris RJ (1984) Developments of a water-maze procedure for studying spatial learning in the rat. Neurosci. Methods 11: 47-60.
- Williams MT, Morford LRL, McCrea AE, Wood SL, Vorhees CV (2002) Administration of D, L-fenfluramine to rats produces learning deficits in the Cincinnati water maze but not the Morris water maze: relationship to adrenal cortical output. Neurotoxicol Teratol 24: 783-796.
- Chow CK, Tappel AL (1972) An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids 7: 518-524.
- Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105: 114-120.
- Haider S, Saleem S, Perveen T, Tabassum S, Batool Z, et al. (2014) Age-related learning and memory deficits in rats: role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. p: 36.
- Ikram H, Ahmad S, Haleem DJ (2011) Effects of apomorphine on locomotive activity and monoamine metabolism: A dose related study. Pak J Pharm Sci 24: 315-321.
- Haleem DJ, Shireen E, Haleem MA (2004) Somatodendritic and postsynaptic serotonin-1A receptors in the attenuation of haloperidol-induced catalepsy. Prog Neuro-Psychopharmacology Biol Psychiatry 28: 1323-1329.
- Pitozzi V, Jacomelli M, Catelan D, Servili M, Taticchi A, et al. (2012) Long-term dietary extra-virgin olive oil rich in polyphenols reverses age-related dysfunctions in motor coordination and contextual memory in mice: Role of oxidative stress. Rejuvenation Res 15: 601–612.
- Haleem DJ, Inam QA, Haleem MA (2015) Effects of clinically relevant doses of methyphenidate on spatial memory, behavioral sensitization and open field habituation: A time related study. Behav. Brain Res 281: 208–214.
- Péczely L, Ollmann T, László K, Kovács A, Gálosi R, et al. (2014) Effects of ventral pallidal D1 dopamine receptor activation on memory consolidation in morris water maze test. Behav. Brain Res 274: 211–218.
- Ikram H, Mushtaq F, Haleem DJ (2014) Dose-dependent effects of tryptophan on learning and memory. Pak J Pharm Sci 27: 1131–1135.
- Zheng A, Li H, Cao K, Xu J, Zou X, et al. (2015) Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem 2015: 190–199.
- Dolu N, Khan A, Dokutan ÃÆââ¬Â¦Ãâà ¾J (2015) Effect of Vitamin E administration on learning of the young male rats. Exp Neurosci 9: 81–85.
- Batool F, Haleem DJ (1997) Neurochemical and behavioural effects of diazepam: evidences from animal models. Pak J Pharm Sci 10: 1–8.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences