Natural Solutions for Gastrointestinal Disorders: The Role of a Nutraceutical Product in Improving Gastrointestinal Function and Well-Being
Esther Garcia1 and Agusti Marti Gil2*
1Marqueta Garcia Pharmacy, Barcelona, Spain 2Greenhouse Data Science, Barcelona, Spain
Published Date: 2024-07-19DOI10.36648/ipctn.9.4.44
Esther Garcia1 and Agusti Marti Gil2*
1Marqueta Garcia Pharmacy, Barcelona, Spain
2Greenhouse Data Science, Barcelona, Spain
- *Corresponding Author:
- Agusti Marti Gil
Greenhouse Data Science, Barcelona,
Spain,
E-mail: agusti@recerca.com
Received date: June 18, 2024, Manuscript No. IPCTN-24-19197; Editor assigned date: June 21, 2024, PreQC No. IPCTN-24-19197 (PQ); Reviewed date: July 05, 2024, QC No. IPCTN-24-19197; Revised date: July 12, 2024, Manuscript No. IPCTN-24-19197 (R); Published date: July 19, 2024, DOI: 10.36648/ipctn.9.4.44
Citation: Garcia E, Gil AM (2024) Natural Solutions for Gastrointestinal Disorders: The Role of a Nutraceutical Product in Improving Gastrointestinal Function and Well-Being. J Nutraceuticals Food Sci Vol.9 No.4: 44.
Abstract
Introduction: Digestion is a critical process that, when disrupted, can lead to gastrointestinal disorders significantly affecting the quality of life. A food supplement is proposed as a therapeutic alternative based on digestive enzymes and natural components such as papaya, fennel and ginger, aimed at alleviating symptoms related to digestive issues.
Methods: A one-week study was conducted with 74 subjects recruited from nine pharmacies. The primary objective was to evaluate the efficacy of the food supplement in study using visual analogue scales, Goldberg subscales for anxiety and somatization and other methods. Subject satisfaction with the product, its safety and adherence to treatment were also examined.
Results: The collected data revealed significant improvements in various gastrointestinal symptoms: Bloating, nausea, vomiting, heartburn, burning, reflux, stomach ache, heavy digestion gas/flatulence and burps. An increase in the quality of life was also observed, corroborated by scales and statistical tests. The product proved to be highly tolerable, with a minimal percentage of subjects experiencing adverse effects. Additionally, statistically significant differences in dietary issues were found when comparing the data with the 2017 Spanish National Health Survey.
Conclusions: The study validated the efficacy of a nutraceutical product in treating a variety of gastrointestinal symptoms. Nearly all subjects tolerated the product well and also showed notable improvements in their quality of life. The research concludes that the product is a viable, effective and well-tolerated treatment option for gastrointestinal disorders, supporting its use in clinical settings to improve both physical symptoms and healthrelated quality of life.
Keywords
Gastrointestinal disorders; Digestive enzymes; Natural components; Food supplement efficacy; Quality of life improvement; Therapeutic alternative
Introduction
Digestion is a complex but essential biological process that turns the food we eat into more basic and manageable substances. This process, carried out by the digestive system, is indispensable for the optimal utilization of nutrients and energy in the foods we consume. In this intricate process, a series of both physical and chemical mechanisms are used to break down food into smaller units, preparing them for absorption and assimilation in the body. However, not all digestion processes are created equal. In some cases, this process can become slow and problematic, causing a series of uncomfortable symptoms that include drowsiness, bloating, stomach ache and reflux.
Gastrointestinal (GI) disorders can affect health-related quality of life and cause physical, mental and social discomfort. This is evident for those who suffer from chronic gastrointestinal symptoms and for the professionals who care for patients with digestive disorders. Patients generally seek care when they have reached a critical point of physical, emotional or social discomfort. Although the range of GI disorders is wide, the digestive tract is surprisingly efficient in its expression of symptoms; these can fall into one of four basic groups: GI pain, gas/bloating, alteration in defecation and upper digestive tract symptoms [1].
The nowadays so-called 'nutraceuticals' have been a fundamental part of healing practices in various cultures since ancient times and continue to be used by some doctors and as home remedies. In particular, they can serve as complementary and alternative medicines for patients with Functional Gastrointestinal Disorders (FGIDs) when primary treatments fail. Since FGIDs often present a wide range of symptoms and can overlap with other conditions, nutraceuticals, which contain diverse components with multiple mechanisms of action, offer a potentially valuable therapeutic pathway. Moreover, their long history of safe use suggests that these options have fewer side effects [2].
The studied product is a food supplement specifically designed to mitigate the challenges associated with slow and problematic digestion. Formulated with a unique combination of natural ingredients and bioactive compounds, this product aims to optimize digestive function and relieve related discomfort [3].
But not only that, it also incorporates the powerful action of papaya, a tropical fruit rich in fiber and low in calories, but perhaps most notable for its content of papain, an enzyme that facilitates the breakdown of proteins [4]. Fennel, another herbal addition to the product, has carminative properties that help expel gas from the digestive tube, reducing bloating and discomfort [5]. Calcium carbonate acts as an effective antacid, neutralizing excess gastric acid in the stomach. Finally, ginger, a root known for its efficacy in preventing nausea, also improves gastric emptying and intestinal transit, contributing to smoother digestion [6].
Together, the ingredients of the nutraceutical product work synergistically to offer a comprehensive solution to digestive challenges, from the initial breakdown of food to the final absorption of nutrients, ensuring a more comfortable and efficient digestion process.
Health-Related Quality of Life (HRQOL) consists of subjective perceptions and objective health status, encompassing physical, psychological and social domains. The evaluation of HRQOL continues to grow in importance, as clinicians and clinical researchers have recognized the impact of Functional Gastrointestinal Disorders (FGIDs). Both generic and diseasespecific Quality of Life (QOL) instruments are used to assess HRQOL [7,8].
Several studies have shown that patients with gastrointestinal disorders, in general, exhibit a higher level of anxiety and general depression, especially those who seek help from gastroenterologists due to their symptoms [9]. This increase in anxiety and depression levels manifests as sleep problems, exhaustion and somatizations, which are the main factors that diminish the quality of life.
Materials and Methods
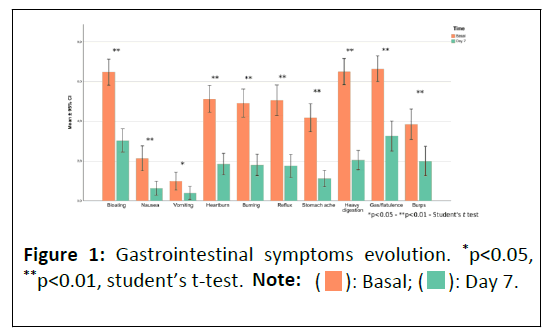
The primary objective of the study was to evaluate the alleviation of gastrointestinal symptoms in patients seven days after beginning their regimen with the studied product (Figure 1). At the baseline visit and again after seven days, patients responded to eleven items using a visual analog scale. These items pertained to various gastrointestinal symptoms, including bloating, nausea, vomiting, heartburn, burning sensation, reflux, stomach pain, heavy digestion, gas/flatulence and belching. The change in symptom scores from the baseline to the final visit was analyzed. The first secondary objective was to assess the improvement in patients' quality of life, for which the GHQ-28 scale was utilized [10-12]. The 14 questions from the GHQ-28 subscales used in this study provided insights into psychosomatic symptoms and anxiety. The second secondary objective was to evaluate the safety and tolerability of the product after seven days of use.
The study population consisted of 74 subjects recruited from nine pharmacies nationwide. Each investigator received a web link or URL that led to an electronic Case Report Form (eCRF). Using assigned usernames and passwords, investigators recorded relevant data. Information was obtained from a single clinical visit with each subject meeting the selection criteria and through a review of their medical history.
The study was conducted in nine pharmacies where subjects with gastrointestinal discomfort were seen. It was designed as a seven-day observational study under normal conditions of use. Subjects included in the study had to be over 18 years old and display some gastrointestinal sign or symptom, in addition to meeting other specific inclusion and exclusion criteria. The inclusion period was estimated to be two months and each pharmacist was responsible for including data for up to 10 subjects who met the requirements. Once informed consent was obtained and it was verified that the subjects met the criteria, pharmacists completed the corresponding sections of the eCRF.
Aquilea Digestivo® is a digestive wellness product designed to address issues caused by inappropriate diet and lifestyle, such as gas, stomach bloating and heartburn. Its composition includes fennel, which helps reduce bloating and gas; papaya, contributing to overall digestive health; ginger, aiding in reducing nausea associated with poor digestion; digestive enzymes, to facilitate digestion and calcium, to neutralize stomach acidity. This product is intended for individuals experiencing digestive discomfort due to their dietary and lifestyle habits, offering a comprehensive solution for improved digestive health.
The treatment under study consisted of the intake of up to two tablets after each meal for a period of seven days. Subjects returned to the pharmacy one week after the initial visit for a review and follow-up.
The necessary sample size for the study was determined using a formula for the Wilcoxon test for repeated measures. A risk alpha level of 0.05 and statistical power of 90% were established. For an effect size of 0.4, 71 cases were required for statistically significant results. However, to anticipate a possible margin of loss between 10% and 15%, the sample was expanded to 80 cases.
As for the variables and measurement instruments, the main variables of assessment were focused on evaluating various symptoms such as bloating, nausea, vomiting, heartburn, burning, reflux, stomach ache, heavy digestion, gas or flatulence and burps. The primary outcome variable recorded was the change in the score of gastrointestinal symptoms from the initial visit to the visit after one week, as the study was designed to last 7 days instead of 4 weeks like in the original model. This measurement was carried out using visual analog scales ranging from 0 to 10 with a precision of 0.1.
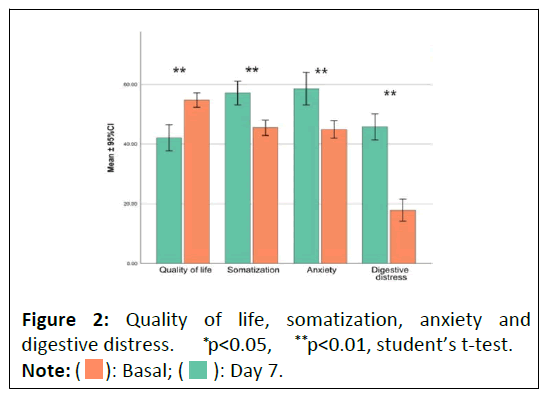
In this study, the Goldberg anxiety and somatization subscales were used to assess the well-being of volunteers. These subscales provide scores expressed as percentages of problems. The sum of the somatization and anxiety subscales provides the overall quality of life score (Figure 2). These two Goldberg scales were chosen to measure quality of life because the study focused on relatively young and healthy volunteers. Moreover, conventional quality of life scales that generally assess functional disability might not have shown the necessary sensitivity to detect changes in this specific context. Quality of life was assessed using the GHQ-28 questionnaire, which assigns scores in a range of 0-100, where a higher score indicates a lower quality of life. To facilitate interpretation, the scores were inverted (100-original score).
Secondary variables that were evaluated included subject satisfaction with the product format, ease of dosing and product size. The safety of the product and the level of subject compliance or adherence to treatment were also studied. These variables provided a more complete view of the product's impact on subjects’ lives and its suitability as a treatment for gastrointestinal discomfort.
A descriptive analysis of all variables collected in the eCRF was performed. Categorical variables were described using frequency lists and proportions. In the case of quantitative variables, central tendency measures such as mean, median and mode, as well as dispersion indices, including standard deviation and minimum and maximum values, were presented. The 25 and 75 percentiles were also calculated to provide a more complete view of the data.
Regarding statistical inference, various tests were used to compare variables between different groups. Continuous variables were represented with the mean and standard deviation, while categorical variables were presented in percentages. Both Cohen's d and odds ratio with 95% confidence intervals were used to assess the effect size for significant results. Differences between visits were examined using repeated measures t-tests or their non-parametric equivalent, the Wilcoxon test, as appropriate. For each subject, two averages were calculated: The average symptoms at the initial visit and the average symptoms one week after starting treatment with the nutraceutical product. These averages were compared using a Student's t-test for repeated measures.
All analyses were performed with a p-value less than 0.05 as the level of significance and the statistical packages SPSS version 26 and R version 4.2.2 were used for the calculations.
Results
In the studied population, Table 1 reveals a significant gender disparity, with 71.8% of the subjects being female and 28.2% male. Furthermore, 50.0% of the sample rated their health as 'good' in the past twelve months, despite 37.2% reporting a chronic or long-term health condition. These data points highlight key demographic and health-related characteristics of the sample.
| Descriptive statistics | n | % | Mean ± SD | |
|---|---|---|---|---|
| Sex | Female | 56 | 71.80% | |
| Male | 22 | 28.20% | ||
| Total | 78 | 100.00% | ||
| Age | 78 | 49.2 ± 15.6 | ||
| Weight | Female | 55 | 69.3 ± 14.4 | |
| Male | 22 | 81.8 ± 15.1 | ||
| Total | 77 | 72.9 ± 15.1 | ||
| Height | Female | 48 | 162.0 ± 6.6 | |
| Male | 19 | 178.1 ± 6.7 | ||
| Total | 67 | 166.5 ± 9.9 | ||
| BMI (kg/cm2) | 67 | 26.5 ± 5.5 | ||
| BMI categories | Severe thinness | 0 | 0.00% | |
| Moderate thinness | 0 | 0.00% | ||
| Mild thinness | 3 | 4.50% | ||
| Normal weight | 26 | 38.80% | ||
| Overweight | 24 | 35.80% | ||
| Mild obesity (type I) | 8 | 11.90% | ||
| Moderate obesity (type II) | 5 | 7.50% | ||
| Morbid obesity (type III) | 1 | 1.50% | ||
| Total | 67 | 100.00% | ||
| Smoker | No | 51 | 65.40% | |
| Yes | 10 | 12.80% | ||
| Ex-smoker | 17 | 21.80% | ||
| Total | 78 | 100.00% | ||
| Frequency of physical activity in leisure time | Does not exercise | 28 | 35.90% | |
| Occasional physical activity/sport | 29 | 37.20% | ||
| Physical activity several times/month | 8 | 10.30% | ||
| Training several times/week | 13 | 16.70% | ||
| Total | 78 | 100.00% | ||
| In the last twelve months, would you say your health has been | Very good | 7 | 9.00% | |
| Good | 39 | 50.00% | ||
| Fair | 26 | 33.30% | ||
| Poor | 6 | 7.70% | ||
| Very poor | 0 | 0.00% | ||
| Total | 78 | 100.00% | ||
| Do you have any chronic or long-term health issues? | Yes | 29 | 37.20% | |
| No | 49 | 62.80% | ||
| Total | 78 | 100.00% | ||
Table 1: Sample characteristics.
It was observed that the average severity of gastrointestinal symptoms significantly decreased from 4.62 at the initial visit to 1.78 at the follow-up visit, representing a 61.5% improvement. The effect size, calculated using "Cohen's d" was 1.8 with a 95% confidence interval (1.33-2.27) and a p-value less than 0.00001. 88% of the subjects showed improvement in their average gastrointestinal symptoms.
The findings revealed that for bloating, there was a 53.17% decrease in symptom severity and 90.1% of patients showed improvement. Nausea saw a reduction of 70.83% in symptoms, with 86% of patients experiencing relief. Vomiting symptom severity decreased by 61.17%, benefiting 78.8% of the patients. Heartburn and burning sensations were reduced by 64.01% and alleviation. Heavy digestion symptoms decreased by 68.70%, with a notable 94.4% of patients experiencing improvement. The symptoms of gas or flatulence saw a 51.20% reduction, aiding 88.7% of the affected individuals. Lastly, burping was reduced by 50.12%, with 80.3% of patients noting an improvement. These results offer valuable insights into the efficacy of the treatments provided for these gastrointestinal disturbances as shown in Table 2.
| Variable | Basal mean (SD) | Final mean (SD) | Average symptom reduction after 7 days (%) | Cohen’s d | IC 95% | P |
|---|---|---|---|---|---|---|
| Bloating | 6.47 (2.94) | 3.03 (2.53) | 53.17 | 3.46 | 0.72, 1.27 | <0.0001 |
| Nausea | 2.16 (2.78) | 0.63 (1.53) | 70.83 | 3.02 | 0.26, 0.74 | <0.0001 |
| Vomiting | 1.03 (2.04) | 0.4 (1.44) | 61.17 | 2.48 | 0.02, 0.48 | 0.031 |
| Heartburn | 5.14 (2.98) | 1.85 (2.34) | 64.01 | 3.21 | 0.74, 1.30 | <0.0001 |
| Burning | 4.92 (3.13) | 1.81 (2.37) | 63.21 | 3.47 | 0.63, 1.16 | <0.0001 |
| Reflux | 5.18 (3.41) | 1.75 (2.53) | 66.22 | 3.71 | 0.65, 1.19 | <0.0001 |
| Stomach ache | 4.11 (3.04) | 1.12 (1.79) | 72.75 | 3.25 | 0.65, 1.19 | <0.0001 |
| Heavy digestion | 6.55 (2.86) | 2.05 (2.1) | 68.7 | 3.38 | 1.02, 1.64 | <0.0001 |
| Gas/flatulence | 6.66 (2.91) | 3.25 (3.29) | 51.2 | 3.65 | 0.66, 1.21 | <0.0001 |
| Burps | 4.01 (3.36) | 2 (3.22) | 50.12 | 3.84 | 0.28, 0.76 | <0.0001 |
Table 2: Improvement of intestinal symptoms from day 0 to day 7.
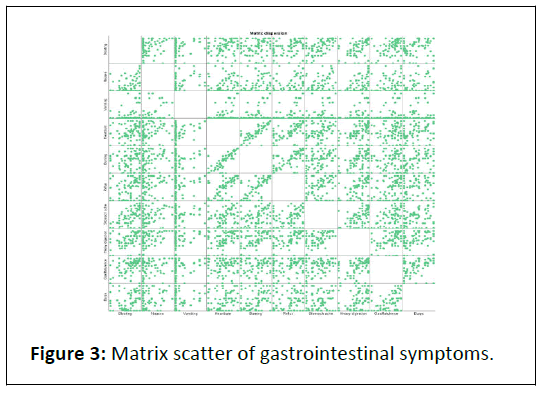
The study revealed that various gastrointestinal symptoms are interconnected, suggesting that a single product might effectively treat multiple symptoms. This conclusion is based on a correlation matrix which demonstrated significant associations among symptoms like bloating, nausea, vomiting, 63.21% respectively, with 85.3% and 84.1% of patients reporting improvements. Reflux symptoms decreased by 66.22%, leading to 86.4% of patients feeling better. Stomach aches had a significant reduction of 72.75%, with 90.6% of patients reporting reflux, stomach ache and gas/flatulence. These findings indicate a pattern of symptom interrelation, supporting the potential of a multi-symptom treatment approach (Figure 3).
At the baseline visit, the average quality of life score was 42.38, which increased to 54.76 at the final visit, reflecting a 29.2% improvement. The adjusted Cohen's d effect size with a 95% confidence interval was 1.15 (0.75-1.55), with a p-value of p<0.00001, as per the paired t-test (n=75). Additionally, 74.67% of the subjects experienced significant improvement in their quality of life, confirmed by a p-value of p<0.05 in the Wilcoxon test.
The research compared the current study sample with data from the 2017 Spanish National Health Survey on three eating problem indicators. The study sample exhibited a higher average in "excess of non-recommended foods" and "deficiency of necessary foods," as well as in the "overall eating problems" indicator, compared to the National health survey results. These differences were statistically significant, indicating notable disparities in eating habits and problems between the two groups.
The study assessed the safety and tolerability of a product and found that 98.67% of participants (76 out of 78) tolerated it well. Only 1.28% (one participant) reported vomiting as an adverse effect. Additionally, 5.13% of the sample (4 out of 78 subjects) experienced some adverse effects. Among these, two subjects had been using the product for 3 days and the others for 4 days. All adverse effects were classified as "Not severe." The subject who reported vomiting experienced an increase in symptoms severity, as indicated by a score escalation from 2 to 10 during a follow-up visit after 7 days.
Discussion
The study demonstrated that the nutraceutical product significantly improved various gastrointestinal symptoms over one week of treatment. A majority of subjects experienced a marked reduction in symptoms such as heavy digestion, nausea, bloating, heartburn, burning, stomach pain, reflux, vomiting and burps. Notably, nine out of ten subjects reported significant improvements in heavy digestion and nausea, indicating the product's effectiveness in treating persistent and debilitating symptoms. Bloating relief was observed in eight out of ten subjects, while over eight out of ten subjects experienced improvements in heartburn, burning, stomach pain, reflux and vomiting. Even less common symptoms like burps showed significant relief in seven out of ten subjects. These results suggest the product's comprehensive efficacy in managing a range of gastrointestinal discomforts, underlining its utility as a versatile treatment option.
The product effectively addresses a broad spectrum of gastrointestinal symptoms, demonstrating statistically significant efficacy. Its impact on improving quality of life within a short span of one week highlights its potential as a substantial therapeutic option in gastrointestinal disorder management. The study also revealed significant correlations among various gastrointestinal symptoms, suggesting complex interactions between them. Notably, symptoms like heartburn and burning, as well as bloating and gas/flatulence, exhibited strong correlations, potentially pointing to common underlying causes or interconnected mechanisms. Nausea and vomiting were significantly correlated with numerous other symptoms, indicating their role as general indicators of gastrointestinal dysfunction. These correlations offer valuable insights for identifying specific clinical syndromes and could guide future research in understanding the causes and relationships of gastrointestinal symptoms. This knowledge is essential for improving both diagnosis and treatment strategies in gastrointestinal health.
The study, utilizing the GHQ-28 questionnaire, indicated a notable improvement in quality of life among the participants, highlighted by significant changes in average scores and a large effect size. This underscores the clinical importance of the study's findings. Additionally, a considerable number of subjects reported improvements in their quality of life, further emphasizing the significance of these outcomes. These results suggest that the interventions employed had a meaningful and positive impact on the participants' quality of life.
The introduction of the Visceral Sensitivity Index (VSI) adds a valuable dimension to the assessment of individuals with gastrointestinal disorders. The VSI evaluates aspects such as concern, fear, vigilance, sensitivity and avoidance relating to the digestive system, particularly focusing on Gastrointestinal Symptom-specific Anxiety (GSA). This tool can enhance our understanding of how interventions affect not just the physical symptoms but also the emotional and psychological well-being of subjects.
Furthermore, the study sample demonstrated dietary habits that differed significantly from those in the Spanish National Health Survey, with a higher tendency towards consuming nonrecommended foods and a deficit in necessary foods. Despite these less optimal dietary habits, the product's effect on symptom improvement was both statistically significant and notable. This finding highlights the importance of addressing dietary issues in this group, considering the implications for health and well-being. The combined use of GHQ-28 and VSI offers a comprehensive perspective on the effectiveness of interventions on both the quality of life and emotional health of individuals with gastrointestinal disorders.
The study indicates that the product was well-tolerated by most subjects, with a minority reporting non-severe adverse effects, including one case of increased vomiting severity during follow-up. These findings suggest the product's general safety and tolerability, though individual responses vary. Additionally, antacids were the most commonly used prior treatment for digestive discomfort, followed by natural preparations, influenced by factors like advertising, medical prescriptions and self-prescription. The product also exhibited a high repurchase intention among subjects.
Conclusion
The research conclusively demonstrates that the product effectively alleviates a range of gastrointestinal symptoms within a week, enhancing overall quality of life as measured by the GHQ-28 questionnaire. Notable correlations among symptoms suggest complex interrelated mechanisms, offering directions for future research. The study also highlighted dietary pattern disparities compared to the Spanish National Health Survey, underscoring the need for dietary considerations in this demographic. With minimal adverse reactions reported, the product emerges as a promising and well-tolerated therapeutic option for gastrointestinal disorders.
References
- Spiegel BM, Khanna D, Bolus R, Agarwal N, Khanna P, et al. (2011) Understanding gastrointestinal distress: A framework for clinical practice. Am J Gastroenterol 106: 380-385.
[Crossref] [Google Scholar] [Indexed]
- Kim YS, Kim JW, Ha NY, Kim J, Ryu HS (2020) Herbal therapies in functional gastrointestinal disorders: A narrative review and clinical implication. Front Psychiatry 11: 601.
[Crossref] [Google Scholar] [Indexed]
- Ianiro G, Pecere S, Giorgio V, Gasbarrini A, Cammarota G (2016) Digestive enzyme supplementation in gastrointestinal diseases. Curr Drug Metab 17: 187-193.
[Crossref] [Google Scholar] [Indexed]
- Muss C, Mosgoeller W, Endler T (2013) Papaya preparation (Caricol®) in digestive disorders. Neuro Endocrinol Lett 34: 38-46.
- Badgujar SB, Patel VV, Bandivdekar AH (2014) Foeniculum vulgare mill: A review of its botany, phytochemistry, pharmacology, contemporary application and toxicology. Biomed Res Int: 842674.
[Crossref] [Google Scholar] [Indexed]
- Ernst E, Pittler MH (2000) Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials. Br J Anaesth 84: 367-371.
[Crossref] [Google Scholar] [Indexed]
- Choi MG, Jung HK (2011) Health related quality of life in functional gastrointestinal disorders in Asia. J Neurogastroenterol Motil 17: 245-251.
[Crossref] [Google Scholar] [Indexed]
- Fuchs KH, Musial F, Eypasch E, Meining A (2022) Gastrointestinal quality of life in gastroesophageal reflux disease: A systematic review. Digestion 103: 253-260.
[Crossref] [Google Scholar] [Indexed]
- Jerndal P, Ringström G, Agerforz P, Karpefors M, Akkermans LM, et al. (2010) Gastrointestinal-specific anxiety: An important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil 22: e646-e179.
[Crossref] [Google Scholar] [Indexed]
- Goldberg DP, Hillier VF (1979) A scaled version of the general health questionnaire. Psychol Med 9: 139.
- Lobo A, Pérez-Echeverría MJ, Artal J (1986) Validity of the scaled version of the General Health Questionnaire (GHQ-28) in a Spanish population. Psychol Med 16: 135-140.
[Crossref] [Google Scholar] [Indexed]
- Campodarbe FRD, Rossello LRP, Porras DGR, Torra BA, Vall IP (1999) Psychometry of anxiety, depression and alcoholism in primary care. Semergen 25: 209-225.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
 ): Basal; (
): Basal; (  ): Day 7.
): Day 7.