Monitoring the Safety of Nutraceuticals through Pharmacovigilance Programme of India
Kalaiselvan V, Archana Saurabh, Pranay Kumar and Singh GN
Kalaiselvan V, Archana Saurabh*, Pranay Kumar and Singh GN
Indian Pharmacopoeia Commission, National Coordination Centre-Pharmacovigilance Programme of India, Ministry of Health and Family Welfare, Govt. of India, Ghaziabad, Uttar Pradesh, India
- *Corresponding Author:
- Archana Saurabh
Indian Pharmacopoeia Commission
National Coordination Centre-Pharmacovigilance Programme of India
Ministry of Health and Family Welfare
Govt. of India, Sector-23, Raj Nagar
Ghaziabad, Uttar Pradesh, India
Tel: +91-9711469037
E-mail:arpharmaarchana@gmail.com
Received: January 25, 2016; Accepted: April 01, 2016; Published: April 04, 2016
Keywords
Pharmacovigilance; Adverse Drug Reaction; Nutraceuticals
Introduction
According to World Health Organization (WHO) Adverse Drug Reaction (ADR) defined as “A response which is noxious and unintended, and which occurs at doses normally used in humans for the prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function. This is of major concern now a day to monitor the safety and adverse drug reactions (ADRs) associated with the use of the Nutraceuticals”[1].
In order to monitor the adverse event associated with the use of medicines Ministry of Health and Family Welfare, Government of India launched a nation-wide programme in the year 2010 to safeguard the public health in India. Indian Pharmacopoeias commission is functioning as National Coordination Centre (NCC) for reporting and monitoring of Adverse Drug Reactions [2]. Adverse Drug Reaction monitoring Centres (AMCs) has been set up in medical institutions including corporate hospitals across India, which plays a vital role in collection and follow up of received ADR reports from patients/consumers. Currently 179 AMCs are functioning under PvPI all over the country [3].
The term Nutraceutical coined by Dr. Stephen De Felice; chairman of the Foundation of Innovation Medicine (FIM) Crawford, New Jersey, in the year 1989 which includes the terms "Nutrition" and "Pharmaceutical” and defined as any substance that is a food or a part of food and provides medical or health benefits, including the prevention and treatment of disease”[4]. Herbal products, isolated nutrients, dietary supplements and diets to genetically engineered ''designer'' foods and processed products such as cereals, soups and beverages may considered as nutraceuticals. Many of these products may possess pertinent physiological functions and valuable biological activities [5].
Nutraceuticals has been expanded to include vitamins, minerals, amino acids, herbs and other botanicals and any dietary substances after the formation of Dietary Supplement Health and Education Act in the year 1994. Due to the multiple therapeutic benefits of nutraceuticals its use has increased worldwide beside the adverse effects associated with its use specially when consumed in large quantities. Lack of proper regulation of nutraceuticals in the pharmaceutical industry and easy availability of nutraceuticals and its regulation has emerged the need to monitor the adverse effect associated with use in public health [4]
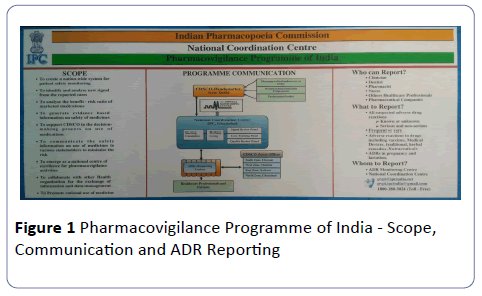
Pharmacovigilance Programme of India encourages all healthcare professionals and consumers to report ADRs associated with Nutraceuticals. The submitted ADR reports are reviewed and analysed at NCC and finally submitted to World Health Organization - Uppsala Monitoring Centre (WHO-UMC), Sweden. The process of reporting and monitoring of ADRs includes the following (Figure 1).
Who can Report?
All Healthcare professionals (Clinicians, dentists, pharmacists, nurses) and non-healthcare professionals including patients/consumers can report ADRs. The pharmaceutical companies can also send the ADRs report for their products to NCC.
What to Report?
PvPI encourages reporting of all types of suspected adverse reactions with all pharmaceutical products irrespective of whether they are known or unknown, serious or non-serious and frequent or rare.
Why to report?
As a healthcare professional it is a moral responsibility to report adverse reactions associated with pharmaceutical products to safeguard public health.
How and Whom to Report?
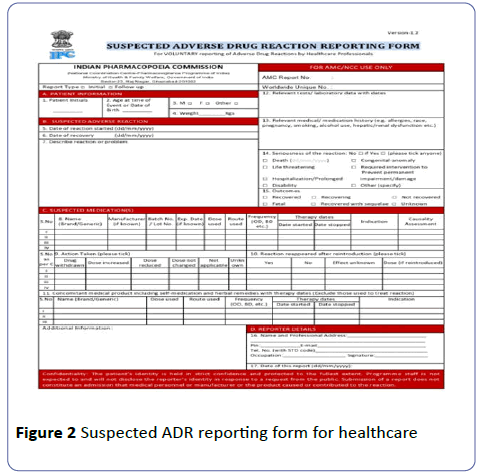
• The ADRs can be reported by filling the Suspected ADR reporting form (Figures 2 and 3).
For healthcare professionals and medicine side effect reporting form (for consumers); which are available on the website of IPC as well as Central Drugs Standard Control Organization (CDSCO). To remove language barrier in ADR reporting consumer reporting form are made available in 10 vernacular languages (Hindi, Tamil, Telugu, Kannada, Bengali, Gujarati, Assamese, Marathi, Oriya, and Malayalam). The filled ADR reporting form can be submitted/send directly to the NCC or to the nearest AMCs or can be directly mailed to pvpi@ipcindia.net or pvpi.ipcindia@gmail.com
• ADRs can also be reported through Helpline number (1800-180-3024) on Monday to Friday from 9:00 am to 5:30 pm. The mobile Android application for reporting of ADRs has also been made available for the public which can be downloaded from Google Play store.
The submitted ADR report does not have any legal implications. The identity of the patients are held in strict confidence and protected to the fullest extent. The submission of a report doesn’t constitute an admission that medical personnel or the product caused or contributed to the reaction.
References
- Brower V (1998) Nutraceuticals: poised for a healthy slice of the healthcare market? Nat Biotechnol 16: 728-731.
- Kalaiselvan V, Saurabh A, Kumar Ranvir, Singh GN (2015) Adverse reactions to herbal products: An analysis of spontaneous reports in the database of the Pharmacovigilance Programme of India. Journal of herbal medicine 5: 48-54.
- https://ipc.nic.in/index1.asp?EncHid=&lang=1&linkid=75&lid=254.
- Bhowmik D, Gopinath H, Pragati KB, Duraivel S, Sampath Kumar KP (2013) Nutraceutical–A Bright Scope and Opportunity of Indian Healthcare Market. The Pharma Innovation 1.
- Srividya AR, Venkatesh N, Vishnuvarthan VJ (2015) Nutraceutical as Medicine, Pharmanest. An International Journal of Advances in Pharmaceutical Sciences 6.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences