Increased Absorption of Palmitoylethanolamide Using a Novel Dispersion Technology System (LipiSperse)
David Briskey*, Alistair R Mallard and Amanda Rao
DOI10.36648/nutraceuticals.5.2.3
David Briskey1,2*, Alistair R Mallard1,2 and Amanda Rao2
1School of Human Movement and Nutrition Sciences, The University of Queensland, St Lucia, QLD, Australia
2RDC Clinical Pvt. Ltd., Newstead, QLD, Australia
- *Corresponding Author:
- David Briskey
School of Human Movement and Nutrition Sciences
Level 2, Connell Building, Blair Drive
The University of Queensland
St Lucia, QLD, 4072, Australia
E-mail: d.briskey@uq.edu.au
Received Date: April 14, 2020; Accepted Date: April 25, 2020; Published Date: May 03, 2020
Citation: Briskey D, Mallard AR, Rao A (2020) Increased Absorption of Palmitoylethanolamide Using a Novel Dispersion Technology System (LipiSperse®). J Nutraceuticals Food Sci Vol.5 No.2:3. DOI: 10.36648/nutraceuticals.5.2.3
Abstract
Title: A Pharmacokinetic study showing the increased absorption of palmitoylethanolamide using LipiSperse®.
Background: Palmitoylethanolamide (PEA) is a naturally occurring endogenous fatty acid that benefits human health by exerting a variety of biological functions related to chronic pain and inflammation. The aim of this trial was to determine whether the use of a novel crystalline dispersion technology, LipiSperse®, can be successfully used to improve the absorption of PEA.
Method: A parallel, double-blind, absorption study to measure uptake of PEA over a 4-hour period. The study was conducted with 28 healthy male and female volunteers over 18 years old. Participants were randomised into 2 groups. One group consumed a single 300 mg dose of PEA together with the LipiSperse® delivery technology (commercially referred to as Levagen Plus), while the other group consumed a single 300 mg dose of unprocessed PEA. Blood samples were taken at baseline and 30, 45, 60, 70, 90, 120, 180, 240 minutes post ingestion. The primary outcome measure of the trial was the change in plasma uptake of PEA over a 4 hour period with the resulting Area Under Curve (AUC), concentration max (Cmax) and maximum change from baseline (Delta Cmax) calculated.
Findings: The Levagen Plus formulation significantly increased plasma PEA concentration above baseline concentrations by 1.75 times that of the standard formulation (p<0.05). The maximum concentration of PEA was observed at 45 minutes post ingestion.
Conclusion: These results indicate that by using the LipiSperse® delivery system, PEA absorption is increased above the standard formulation.
Keywords
Palmitoylethanolamide; Bioavailability; LipiSperse; Dispersion technology; Absorption; Drug delivery
Abbreviations
AUC: Area Under Curve; BSTFA: Bis-(trimethylsilyl) Trifluoroacetamide; Cmax: Concentration Max; D8-AA: D8-Arachidonic Acid; DIPEA: Di-Isopropylethylamine; Cmax: Maximum Change From Baseline Delta; XPEA: Pentafluorobenzylbromide; PFBBr: Pentafluorobenzylbromide; SE: Standard Error; TMCS: Trimethylchlorosilane
Introduction
Palmitoylethanolamide (PEA) is an endogenous saturated fatty acid derivative. In the body, PEA is synthesized from palmitic acid (C16:0), the most common fatty acid. Synthesis of PEA takes place in membranes of various cell types, is produced on demand and acts locally. When cells are subjected to potentially harmful stimuli, they express a selective enzyme that releases PEA from the membrane.
Since its discovery in the 1950s, PEA has been widely studied for its anti-inflammatory and analgesic properties. PEA is reported to act by down regulating mast cell degranulation at local sites and therefore exerts an antagonistic action against inflammation and pain receptor stimulation [1]. Since 1970, the anti-inflammatory and other immune-modulating properties of PEA have been shown placebo-controlled double-blind clinical trials [2].
In addition to its anti-inflammatory activity, PEA also produces analgesia, neuroprotection, and possesses antiepileptic properties [3-19]. The mechanism by which tissue levels of PEA are regulated is largely unknown. Studies indicate that PEA accumulates during cellular stress (e.g tissue injury and inflammation). For example, PEA has been shown to increase in the brain following an ischemic event and even death, as well as in response to ultraviolet-B irradiation in mouse epidermal cells [20-22]. The proposed antiinflammatory effects of PEA is reported to act via the LPSstimulated pathway inhibiting the secretion of leptin [23].
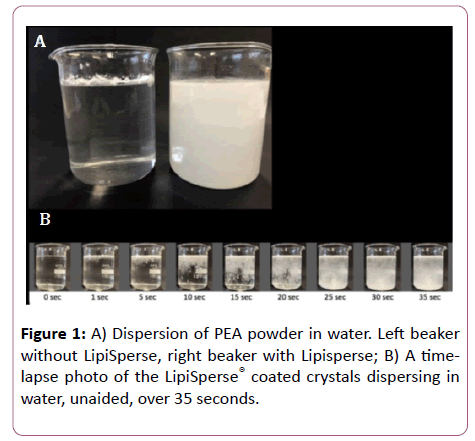
The present study aimed to compare the bioavailability of a single dose of commercially available PEA (LevagenTM) with a PEA+LipiSperse® delivery complex (LevagenTM+). As previously described, LipiSperse® is a novel delivery system designed to increase the dispersion of lipophilic agents in aqueous environments [24]. The addition of lipophilic active ingredients often, leads to decreased active load in final formulations. LipiSperse® is a mixture of surfactants, polar lipids and solvents that allows PEA to disperse in water (Figure 1A). Once dispersed in water, LipiSperse® then goes on to prevent the PEA crystals from agglomerating. Prevention of agglomeration in turn leads to increased specific surface area of PEA in the gastro-intestinal tract, theoretically improving absorption (Figure 1B).
Methods
Study design and procedures
A single equivalent dose, randomised, double-blinded study was used to evaluate the bioavailability of 2 different PEA formulations administered in single 300 mg doses. Participants were allocated to 1 of 2 groups Group 1 mg-300 mg PEA (LevagenTM+), Group 2 mg-300 mg standard PEA (LevagenTM). LevagenTM was supplied by Gencor Pacific Ltd Hong Kong and LipiSperse® is a patent pending technology supplied by Pharmako Biotechnologies Pty Ltd, Sydney Australia. This study was conducted in accordance with ethical approval from Bellberry Limited. All participants provided written informed consent and screened for inclusion and exclusion criteria.
Subjects
Subjects were adult male (n=11) and female (n=17) volunteers between the ages of 18-30 years. All participants were in normal physical health (BMI<25) as assessed through subject screening (e.g medication use). Excluded were participants with any clinically significant medical condition, use within the past 3 months of test nutrients and/or antioxidants; current use of prescription medications except the oral contraceptive pill if female; and known allergy to any test nutrient and/or antioxidant.
All participants were advised to fast until after the collection of the first blood sample. This is a standard feeding study with nutritionally balanced meals and snacks provided during the sample collection. Subjects remained on site for the full 4 hours of sample collection. While at the research centre, subjects were monitored and asked to report any side effects experienced.
Bioanalysis
For PEA bioavailability analysis, blood samples (3 mL collected into ethylenediaminetetraacetic acid containing tubes) were drawn prior to supplementation (hour 0) and at 30, 45, 70, 90, 120, 180, and 240 minutes post supplementation. Once obtained, the blood collection tube was briefly mixed by inversion, placed on ice and centrifuged within 10 minutes of collection (600 xg, 4°C for 10 minutes) to separate the plasma. Once spun, plasma was carefully aliquoted and stored at -80°C.
Sample extraction
Plasma samples were removed from storage at -80°C and allowed to thaw to room temperature. Once thawed, 100 μL of sample was added to a microfuge tube along with 20 μL of an internal standard solution (50 ng/mL of D8-arachidonic acid (D8-AA) in ethanol). Proteins were precipitated by adding 100 μL of acetone, vortex mixing for 15 seconds and put on ice for 10 minutes. The resulting solution was spun at 12,000 xg for 10 minutes before the supernatant was removed into a new tube. To the supernatant, 800 μL of a methanol/chloroform solution (2:1) was added along with 240 μL of 3M HCl to achieve phase separation. This solution was vortex mixed for 10 seconds followed by gentle mixing on a rotator. After 10 minutes of gentle rotation, the tubes were centrifuged at 12,000 xg for 10 minutes with the resulting chloroform layer (bottom layer) transferred to a glass culture tube and dried under a stream of nitrogen gas. Once dry, the samples were reconstituted in 100 μL of ethanol, mixed and the contents transferred to salinized GC-MS glass inserts and dried under nitrogen. Dried samples were derivatized via the addition of 40 μL of pentafluorobenzylbromide (PFBBr, 10% in acetonitrile -4 μL of PFBBr and 36 μL of ACN) and 20 μL diisopropylethylamine (DIPEA, 10% in acetonitrile -2 μL DIPEA and 18 μL of ACN) and vortex mixed for 5 seconds. Samples were then incubated at room temperature for 30 min before being dried under nitrogen and the insert placed into GC-MS vials. To each vial, 10 μL of anhydrous pyridine and 20 μL of bis-(trimethylsilyl) trifluoroacetamide and trimethylchlorosilane (BSTFA+TMCS, 99:1) was added, the vial capped and vortex mixed for 5 seconds. The samples were incubated for 20 min at 45°C. The samples were allowed to cool before 70 μL of anhydrous hexane was added and the samples place on the auto sampler rack for analysis.
Standard
PEA was purchased from Sigma Aldrich (P0359-10MG) and stored at -20°C as per manufacturer’s instructions. The PEA standard was made up to a 1 mM solution with ethanol. Working standard solutions were prepared by diluting the 1 mM solution with hexane for 500 pmol/mL, 100 pmol/mL, 50 pmol/mL, 10 pmol/mL and 1.0 pmol/mL solutions. Ethanol was initially used as a diluent for the stock solution due to the concentration of PEA that can be dissolved into it. Hexane was used as a diluent for all working standards as it is better suited for GC-MS injections.
GC-MS
The GC-MS method used for the analysis of samples was developed based on several existing method for PEA analysis [25-27]. Samples were analysed for PEA concentration using a Varian 320 MS/MS, with a Varian 450 gas chromatograph equipped with a CP8400 auto sampler. 1 μL of sample was introduced in split-less mode using a Hamilton syringe. After 1 minute the injector port was switch to a 1:20 split. The injector operated at 250°C with an SGE Analytical Science column (BP5 30 m × 0.25 mm ID, Film=0.25 μM) with helium as the carrier gas at a flow of 1 mL/min. The column was started at 100°C and held for 1 minute before increasing to 300°C at a rate of 40°C/minute where it was then held for 9 minutes for a total run time of 15 minutes.
Bioavailability parameters and analysis
Bioavailability parameters were analysed using GraphPad Prism 7. Due to endogenous PEA, Area under the Curve (AUC) data was calculated as a change from baseline and any negative value was given a value of “0” for analysis. The AUC and maximum concentration (Cmax) was calculated for each participant individually and averaged per group. Differences between groups for the Cmax and AUC were analysed using a parallel group two-tail t-test at a significance set to below 0.05. All statistics and concentrations presented are arithmetic mean data ± standard error (SE).
Results
All 28 people recruited (n=14 per group) completed the study. The average participant age for group 1-LevagenTM+ (n=14) was 27.6 ± 4.8 years and group 2-Standard PEA (n=14) was 28.1 ± 4.9 years. All biological samples for PEA fell within the linear standard curve with an intra-assay precision CV of 4.8% and inter-assay variability and precision CV of 7.3%. No adverse events were reported during the study.
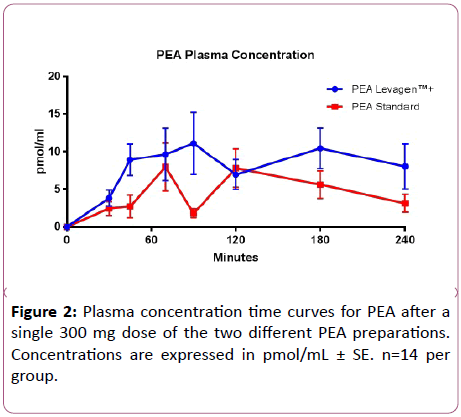
PEA supplementation significantly increased total AUC in both groups (p<0.05), with LevagenTM+ significantly increasing AUC compared with the standard formulation (p<0.05; Figure 2 and Table 1). PEA supplementation increased Cmax concentration from baseline in only the LevagenTM+ group (p<0.05; Table 1). PEA concentration at baseline was not significantly different between the two groups (Table 1).
| Plasma PEA concentrations | ||
|---|---|---|
| Group 1 LevagenTM+ 300 mg | Group 2 Standard PEA 300 mg | |
| Baseline (pmol/mL) | 11.9 ± 4.55 | 15.2 ± 4.25 |
| Delta Cmax (pmol/mL) | 11.12 ± 4.13* | 7.96 ± 3.19 |
| Peak timing (min) | 105 | 125 |
| Total AUC (0-4h) | 1,942 ± 701.1# | 1,117 ± 485.1 |
*Significant compared to baseline value in the same group; #Significant compared to standard PEA group p<0.05
Table 1: Plasma PEA concentrations for both groups. Total AUC is calculated on the PEA concentration change from baseline data.
Discussion
To date, there is limited data published on the bioavailability of PEA in human plasma. As such, it is difficult to compare these results to any other publication. Rather, it serves as a means to complement existing literature that shows the potential benefits of PEA. At present, there is evidence supporting the beneficial effects of PEA supplementation for the treatment of conditions associated with inflammation [2]. However, as with most lipid-based supplements, PEA traditionally has shown poor absorption in animal models [28,29] and this may limited its potential use and/or efficacy. By increasing the absorption of PEA, as presented here, there is the potential for increasing the efficacy of PEA in conditions associated with inflammation. Numerous strategies are currently used to improve the absorption of lipid based supplements, such as PEA, which include, but is not limited to: emulsification [5] and micronized dispersion [30,31]. However, due to the numerous variabilities in each product and delivery mechanism, it is difficult to compare many of the findings reported in the literature. However, the overarching results of existing literature indicate PEA is an important molecule in the body and its potential benefits as a supplement are evident.
An example of the difficulty in comparing literature is a manuscript by Petrosino and colleagues [29] who conducted a study using both dogs and humans. Their trial in humans showed similar Cmax results to those presented here, with a 2- fold increase in peak plasma PEA concentration using a 300 mg dose of PEA in a micronized form. Whether the overall bioavailability of the two studies is comparable, however, is difficult to assess. While the present paper shows plasma PEA remains elevated above baseline even 4 hours after supplementation, Petrosino and colleagues [29] showed a return to baseline within 4 hours. These results demonstrates the importance of the presented delivery system and potentially the importance of the PEA form used.
The current study, examined the effect of LipiSperse®, a novel delivery system that uses dispersion technology to enhance the absorption of hydrophobic agents, on the absorption of a commercially available PEA formulation (LevagenTM). We have previously shown a similar LipiSperse® formulation is able to increase the absorption of curcumin [32]. The present trial was conducted under standardized conditions with the aim of controlling exogenous PEA both prior to, and during the investigation. As consumption of different foods, particularly fats, can increase the absorption of supplements, all trial participants consumed the same foods on the day of the trial. Baseline concentrations reported in this trial are similar between each group and the reported values are consistent to other reported PEA plasma values [33].
Following supplementation with a single 300 mg dose of PEA, LevagenTM+ elicited the greatest increase, with total PEA plasma AUC increasing by 1.7-fold (p<0.05) compared to the standard product. The pre-epithelial aqueous barrier of the gastrointestinal lumen is one of the major limiting factors for absorption of orally dosed hydrophobic supplements. LipiSperse® coats the surface of the PEA molecule, reducing the hydrophobic nature of PEA and acting as a dispersing agent and likely responsible for the increase in gastrointestinal absorption, as reported here, potentially due to the prevention of agglomeration.
There was no statistically significant difference between the Cmax of the two compounds, however, the LevagenTM+ formulation was able to maintain a consistently higher plasma concentration compared to the standard formulation (Figure 2). By maintaining a steady state plasma concentration, LevagenTM+ may aid in the treatment of inflammatory conditions by providing a potentially longer, more sustained, treatment period.
The kinetic profile of PEA indicates a two peak plasma concentration-time course over the 4 hours (90 min and 180 min for LevagenTM+ and 70 min and 120 minutes for standard). Both PEA formulations demonstrated an initial and rapid increase then sharp decrease in plasma concentration followed immediately after by a second peak of equal height (Figure 2). The exact cause of the second peak is unknown. One speculation is that this could represent hepatic recycling, however the rate at which this occurs may make this unlikely. Alternatively, it could be that there is a postprandial effect in the hours following the consumption of breakfast. The decrease between peaks in plasma concentration appears to be delayed and minimized by the LevagenTM+ formulation. The rate of appearance and disappearance of PEA in the plasma supports the role of PEA as a potential compound in the treatment of pain and inflammation. However, further human clinical trials are required to support this theory.
The one limitation of this study is the collection period. As there were no existing human bioavailability studies to go by, we developed the protocol based on a pilot trial conducted (data not published), animal work and the nature of the substance predicted to be fast absorbing. From the initial pilot study, we concluded that the peak of PEA occurred at approximately 90 minutes and had returned to baseline by 3- hours. Therefore, a 4-hour collection was determined to be optimal for the trial. However, the collection of samples over 4-hours appears to be short of what should ideally be collected, as evident by the plasma PEA concentration not having returned to baseline at 4-hours. Had the sample collection been over 6 or 7-hours, we would have likely seen plasma PEA concentrations return to baseline concentrations. The collection of additional data points would likely further increase the advantage shown by LipiSperse®, as the standard formulation appears to be returning to baseline much earlier than the LevagenTM+ group. Therefore, the change in AUC between the two groups over a longer period would increase above the current 1.75 fold increase.
Conclusion
In conclusion, these results indicate that by combining PEA with the LipiSperse® technology, the PEA absorbs more effectively. Additional human clinical trials need to be undertaken to investigate this technology and the compound’s efficacy for maintaining and improving human health.
Ethics
This study was conducted with ethical approval from Bellberry limited (approval number: 2016-04-305-A-6). Further, the authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, written informed consent has been obtained from the participants involved.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This study received funding and product support from Pharmako Biotechnologies and Gencor Pacific.
Competing Interest
The authors declare that no competing interests exist.
References
- De Filippis D, D'amico A, Iuvone T (2008) Cannabinomimetic control of mast cell mediator release: new perspective in chronic inflammation. J Neuroendocrinol 20: 120-125.
- Keppel Hesselink JM, De Boer T, Witkamp RF (2013) Palmitoylethanolamide: A natural body-own anti-inflammatory agent, effective and safe against influenza and common cold. Int J Inflam 2013: 151028.
- Artukoglu BB, Beyer C, Zuloff-Shani A, Brener E, Bloch MH (2017) Efficacy of palmitoylethanolamide for pain: A meta-analysis. Pain Physician 20: 353-362.
- Andresen SR, Bing J, Hansen RM (2016) Ultramicronized palmitoylethanolamide in spinal cord injury neuropathic pain: A randomized, double-blind, placebo-controlled trial. Pain 157: 2097-2103.
- Gabrielsson L, Mattsson S, Fowler CJ (2016) Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br J Clin Pharmacol 82: 932-942.
- Paladini A, Fusco M, Cenacchi T, Schievano C, Piroli A (2016) Palmitoylethanolamide, a special food for medical purposes, in the treatment of chronic pain: A pooled data meta-analysis. Pain Physician 19: 11-24.
- Keppel JM, Kopsky DJ (2015) Palmitoylethanolamide, a neutraceutical, in nerve compression syndromes: Efficacy and safety in sciatic pain and carpal tunnel syndrome. J Pain Res 8: 729-734.
- Costagliola C, Romano MR, Dell'omo R, Russo A, Mastropasqua R, et al. (2014) Effect of palmitoylethanolamide on visual field damage progression in normal tension glaucoma patients: results of an open-label six-month follow-up. J Med Food 17: 949-954.
- Coppola M, Mondola R (2014) Is there a role for palmitoylethanolamide in the treatment of depression? Med Hypotheses 82: 507-511.
- Skaper SD, Facci L, Fusco M (2014) Palmitoylethanolamide, a naturally occurring disease-modifying agent in neuropathic pain. Inflammopharmacology 22: 79-94.
- Strobbe E, Cellini M, Campos EC (2013) Effectiveness of palmitoylethanolamide on endothelial dysfunction in ocular hypertensive patients: a randomized, placebo-controlled cross-over study. Invest Ophthalmol Vis Sci 54: 968-973.
- Gatti A, Lazzari M, Gianfelice V, Di Paolo A, Sabato E (2012) Palmitoylethanolamide in the treatment of chronic pain caused by different etiopathogenesis. Pain Med 13: 1121-1130.
- Marini I, Bartolucci ML, Bortolotti F, Gatto MR, Bonetti GA (2012) Palmitoylethanolamide versus a nonsteroidal anti-inflammatory drug in the treatment of temporomandibular joint inflammatory pain. J Orofac Pain 26: 99-104.
- Truini A, Biasiotta A, Di Stefano G (2011) Palmitoylethanolamide restores myelinated-fibre function in patients with chemotherapy-induced painful neuropathy. CNS Neurol Disord Drug Targets 10: 916-920.
- Pescosolido N, Librando A, Puzzono M, Nebbioso M (2011) Palmitoylethanolamide effects on intraocular pressure after Nd:YAG laser iridotomy: An experimental clinical study. J Ocul Pharmacol Ther 27: 629-635.
- Gagliano C, Ortisi E, Pulvirenti L (2011) Ocular hypotensive effect of oral palmitoyl-ethanolamide: A clinical trial. Invest Ophthalmol Vis Sci 52: 6096-6100.
- Conigliaro R, Drago V, Foster PS, Schievano C, Di Marzo (2011) Use of palmitoylethanolamide in the entrapment neuropathy of the median in the wrist. Minerva Med 102: 141-147.
- Pescosolido N, Puzzono M (2011) First clinical case of effective medical treatment of the vitreoretinal traction with recovery of the visual acuity. Clin Ter 161: e143-e147.
- Calabro RS, Gervasi G, Marino S, Mondo PN, Bramanti P (2010) Misdiagnosed chronic pelvic pain: pudendal neuralgia responding to a novel use of palmitoylethanolamide. Pain Med 11: 781-784.
- Buczynski MW, Parsons LH (2010) Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br J Pharmacol 160: 423-442.
- Berdyshev EV, Schmid PC, Dong Z, Schmid HH (2000) Stress-induced generation of N-acylethanolamines in mouse epidermal JB6 P+ cells. Biochem J 346 Pt 2: 369-374.
- Magina S, Esteves-Pinto C, Moura E (2010) Inhibition of basal and ultraviolet B-induced melanogenesis by cannabinoid CB(1) receptors: A keratinocyte-dependent effect. Arch Dermatol Res 303: 201-210.
- Hoareau L, Ravanan P, Gonthier MP (2006) Effect of PEA on LPS inflammatory action in human adipocytes. Cytokine 34: 291-296.
- Briskey D, Sax A, Mallard AR, Rao A (2019) Increased bioavailability of curcumin using a novel dispersion technology system (LipiSperse(R)). Euro J Nutri 58: 2087-2097.
- Giuffrida A, Rodriguez De F, Piomelli D (2000) Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem 280: 87-93.
- Maccarrone M, Attina M, Cartoni A, Bari M, Finazzi-Agro A (2001) Gas chromatography-mass spectrometry analysis of endogenous cannabinoids in healthy and tumoral human brain and human cells in culture. J Neurochem 76: 594-601.
- Devane WA, Hanus L, Breuer A (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946-1949.
- Vacondio F, Bassi M, Silva C (2015) Amino acid derivatives as palmitoylethanolamide prodrugs: synthesis, in vitro metabolism and in vivo plasma profile in rats. PloS One 10: e0128699.
- Petrosino S, Schiano Moriello A, Cerrato S (2016) The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br J Pharmacol 173: 1154-1162.
- Impellizzeri D, Bruschetta G, Cordaro M (2016) Erratum to: Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J Neuroinflammation 13: 129.
- Evangelista M, Cilli, De Vitis R, Militerno A, Fanfani F (2018) Ultra-micronized palmitoylethanolamide effects on sleep-wake rhythm and neuropathic pain phenotypes in patients with carpal tunnel syndrome: an open-label, randomized controlled study. CNS Neurol Disord Drug Targets 17: 291-298.
- Darmani NA, Izzo AA, Degenhardt B (2005) Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology 48: 1154-1163.
- Artursson P, Karlsson J (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 175: 880-885.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences