Formulation Development and Evaluation of Anti-diabetic Nutraceutical Instant Soup Powder Formulation
Harshita Ghuse*
Department of Nutraceuticals, Institute of Medical Education and Research, Chandigarh, India
- *Corresponding Author:
- Harshita Ghuse
Department of Anaesthesia and Intensive Care,
Institute of Medical Education and Research,
Chandigarh,
India,
Tel: 9158513955;
E-mail: ghuseharshita@gmail.com
Received date: December 15, 2022, Manuscript No. IPCTN-22-15410; Editor assigned date: December 19, 2022, PreQC No. IPCTN-22-15410 (PQ); Reviewed date: January 03, 2023, QC No. IPCTN-22-15410; Revised date: March 17, 2023, Manuscript No. IPCTN-22-15410 (R); Published date: March 24, 2023, DOI: 10.36648/ IPCTN.8.1.003
Citation: Ghuse H (2023) Formulation Development and Evaluation of Anti-diabetic Nutraceutical Instant Soup Powder Formulation. J Nutraceuticals Food Sci Vol:8 No:1
Abstract
The research study was conducted to develop anti diabetic nutraceutical instant soup powder formulation with the use of jamun seed powder, fenugreek seed powder, gurmar leaves powder for anti-diabetic activity and garlic powder, curry leaves powder, black pepper powder, and black salt for spices, and mushroom, tomato powder for flavoring, and figer millet flour for thickening of soup the soup powder in different ratios of the ingredients F1, F2, F3 and F4, the prepared formulations were evaluated for sensory evaluation and result reviles F4 found to be best with overall acceptability, hence F4 select ed for further study The results of nutritional analysis indicate that, formulated instant soup powder was enhanced with protein and carbohydrate and low in fat with high energy value. Antioxidant and antimicrobial content of formulated instant soup powder was found to significant, outcome of alpha amylase inhibition assay indicate that formulated instant soup powder retained low glycemic index and can be supplemented to diabetic patient the shelf–life properties were found to be good and it retained flavor and test after 3 months of storage. Hence, this formulated anti diabetic nutraceutical instant soup powder can be easily manufactured at low cost and it can definitely meet out the need of growing functional food market.
Keywords
Diabetes; Nutraceutical; Instant soup powder; Functional food
Introduction
Diabetes
Diabetes is “one of the largest global health issue in the 21st century” the International Diabetes Federation (IDF) In 2017, estimated 424.9 million people 20–79 years globally are suffering from diabetes where 1 in 11 people are affected by the disease with a prevalence rate of 8.8%; 1 in 2 adults is undiagnosed (212 million); and 12% of global health expenditure is spent on diabetes (USD727 billion [1]. It is the second leading cause of years of life lost to premature death and the fourth leading cause of years lived with disability.
Diabetes mellitus is a metabolic disorder characterised by hyperglycaemia or high blood sugar. The characteristic symptoms of diabetes are polyuria (excessive urine production), polydipsia (thirst and increased fluid intake) and blurred vision; these symptoms may be absent; if the blood sugar is only mildly elevated. The World Health Organisation (WHO) recognises three main forms of diabetes mellitus: Type 1, type 2 and gestational diabetes (occurring during pregnancy), which have similar signs, symptoms and consequences, but different causes and population distributions. Ultimately, all forms are due to the beta cells of the pancreas being unable to produce sufficient insulin to prevent hyperglycaemia [2]. Type 1 is usually due to autoimmune destruction of the pancreatic beta cells, which produce insulin. Type 2 is characterised by tissue wide insulin resistance, but impairment of beta cell function is necessary for its development. Gestational diabetes is similar to Type 2 Diabetes (T2D), in that it involves insulin resistance due to predisposal of pregnancy hormones.
Diabetes is considered in the top five of the most significant diseases in the developed world. Although extensive armamentarium has been developed to combat this ancient disease but now a days, focus has been shifted to identify effective agents that can be used along with developed drug to treat this disease synergistically.
Nutraceutical in treatment of diabetes
Food and drugs from nature are playing a quite significant role in public healthcare system throughout the world [3]. Human inquisitiveness and search for specific constituents of plants, animals, minerals and microbial origin, which are beneficial to our overall health, has coined terms like ‘functional food’ or ‘nutraceuticals’. Dr. Stephen L. Defelice defined nutraceuticals as any substance that may be considered as food or part of food, which in addition to its normal nutritive value; provide health benefits including prevention of disease. The term ‘nutraceuticals’ came from a combination of ‘nutrition’ and ‘pharmaceuticals’ and can be defined as a food or part of a food that provides medical or health benefit including the prevention and treatment of a disease.
Perhaps no other disease is as closely linked to nutrition as diabetes. Although nutrition plays a significant role in its development; it is also one of the most powerful tools in treatment of diabetes. The use of nutritional supplements in the treatment of the diabetes like vitamins, such as vitamin C and B, minerals such as chromium, as well as herbs like Gymnema sylvestre, is well documented as safe and effective way to lower blood sugars as well as for prevention of diabetic complications [4]. More importantly, combined of scientifically validated diabetic formula to work synergistically for effective management of diabetes and related complications.
Instant soup powder
Nutritional rich convenience foods formulation will have definite uprising and satisfies the market demands. Canned foods, convenience foods, frozen foods, dried foods, preserved foods, etc. comes under instant soup powders are similar kind of instant foods which have extended popularity in the recent years, by way of providing convenience, hygienic, extensible shelf life. A balanced nutrition is obtained by including whole cereals, vegetables and pulses in soup formulations.
Materials and Methods
Selection of raw material
Jamun, gurmar powder, fenugreek seed and other spices and ingridient including finger millet powder, tomato powder, garlic, curry leaves, black paper, black salt etc. was collected from local market, oyster mashroom were collected from local cultivator from Kampthee, Nagpur, and stevia from Bhagyashee lab Nagpur.
Processing for fenugreek seed powder
Fenugreek seed were obtained from the residential area of Nagpur, India the processing of fenugreek seed was carried out, washing and draining of seed were carried out and allow to complete drying under the sunlight in sunny days, after complete drying of seed, the grinding of seed carried out in to fine powder, sieving of powder by sieve no. 44 and powder were subjected for defatting, 100 gm of fenugreek seed powder extracted with sufficient amount of petroleum ether for overnight, after that fenugreek seed powder filter out and draining of powder were carried out and the defatted fenugreek seed powder were obtained.
Preparation of spice powder
Firstly pealing of garlic were carried out and washing and draining of garlic and curry leaves were takes place, were then cutting of garlic and curry leaves were carried out and allowed for sundrying in very sunny days till complete drying, after complete drying grinding of individually garlic and curry leaves were carried and passing though sieve (sieve no. 44) to obtained fine powder, after that garlic powder, curry leaves powder, black paper powder, black salt and stevia powder were mix completely to obtained spice powder.
Preparation and formulation of antidiabetic nutraceutical instant soup powder formulation
Antidiabetic nutraceutical instant soup powder mixture prepared by mixing of jamun powder, defatted fenugreek powder, gurmar powder as antidiabetic active herbs with other ingredient tomato powder for flavouring the soup mixture, finger millet flour and mashroom powder for thickening the soup and prepared spice powder, the prepared instant soup powder were then sealed in a translucent or coloured polythene bags and used for further analysis like sensory evaluation, proximate analysis metal content and microbial contamination, etc.
Cooking procedure for prepared instant soup powder
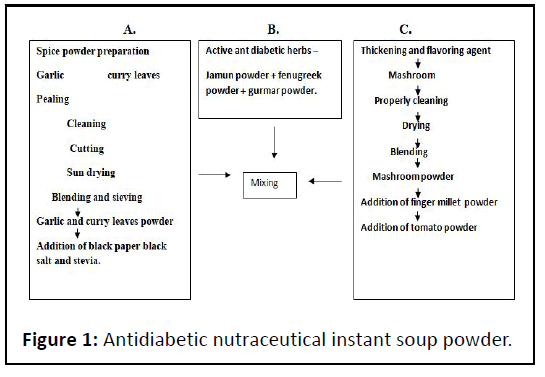
Ttake 100 ml of water and add Ten gram of the prepared soup mix powder in it then allow to boil for 3 to 5 min then Pour in to soup bowl, stir it and serve it hot (Figure 1 and Table 1).
| Batches | F1 | F2 | F3 | F4 | |
|---|---|---|---|---|---|
| Sr. no. | Ingredient (powder) | Quantity (mg) | |||
| 1 | Jamun | 650 | 550 | 450 | 350 |
| 2 | Fenugreek | 650 | 550 | 450 | 350 |
| 3 | Gurmar | 600 | 500 | 400 | 300 |
| 4 | Mashroom | 1000 | 1000 | 1000 | 1000 |
| 5 | Finger millet | 1500 | 1500 | 1500 | 1500 |
| 6 | Tomato | 3000 | 3000 | 3000 | 3000 |
| 7 | Garlic | 1000 | 1000 | 1000 | 1000 |
| 8 | Curry leaves | 500 | 500 | 500 | 500 |
| 9 | Black paper | 500 | 500 | 500 | 500 |
| 10 | Black salt | 1000 | 1000 | 1000 | 1000 |
| 11 | Stevia | 450 | 450 | 450 | 450 |
Table 1: Formula for instant soup powder formulation.
Evaluation of antidiabetic nutraceutical instant soup powder formulation
Physicochemical parameter of formulated instant soup powder: Physicochemical parameter like pH, viscocity, moisture content and ash values were determined according to standard procedure as fallows
Moisture content and ash values for active anti diabetic herb and formulated instant soup powder: Moisture content ash values of the the formulated instant soup powder was determined by standard procedure given by WHO.
pH of formulated instant soup powder: A definite amount of instant soup powder (10 gm) was dissolved in 100 ml water and the pH of formulation was recorded using digital pH Meter.
Viscocity of formulated instant soup powde: The viscosity of the instant soup formulation was measured using Brookfield viscometer the measurement was carried out at 25°C ± 1°C, 0.5 rpm speed using spindle no. 62 in triplicate [5].
Sensory evaluation: In sensory evaluation, each sample was subjected to five point hedonic scale test (5-excellent, 4- very good, 3-good, 2-poor, 1-very poor) and acceptability of sample was judged by 27 untrained members. From the institute the panelists judged the sensory characteristic such as appearance, odor, color, taste, consistency and overall acceptability of the samples.
Nutrient analysis of formulated instant soup powder: Moisture content, ash values and nutrient analysis such carbohydrate, protein, fat, and energy value was evaluated for formulated instant soup AOAC method (2000).
Heavy metal determination of formulated instant soup powder: Lead, arsenic, cadmium and mercury of formulated instant soup power were determined by flame atomic absorption spectrometric method.
Antimicrobial, antioxidant and antidiabetic activity of formulated instant soup power: Antimicrobial activities of formulated instant soup power were determined by agar well diffusion method [6]. Antioxidant activity of formulated instant soup power was analyzed by DPPH assay. Antidiabetic activity of formulated instant soup power and marketed anti diabetic nutraceutical formulation was assessed through α-amylase inhibition assay Methanolic extract of sample was dissolved in DMSO in order to obtain concentrations of 10, 20, 40, 60, 80, and 100 μg/mL. Then, 0.2 mL of sample of particular concentration was added to the tube containing the substrate solution [7]. In addition, 0.1 mL of porcine pancreatic amylase in Tris–HCl buffer (2 units/mL) was added to the tube containing the extract and substrate solution. The reaction was carried out at 37°C for 10 min. The reaction was stopped by adding 0.5 mL of 50% acetic acid in each tube. The reaction mixture was centrifuged at 3000 rpm for 5 min at 4°C. The absorbance of resulting supernatant was measured at 595 nm using spectrophotometer Acarbose, a known α-amylase inhibitor was used as a standard drug. The experiments were repeated thrice. The α-amylase inhibitory activity was calculated by using following formula.

Results and Discussion
Physicochemical parameter
Moisture content and ash values: The physicochemical quality standardization like moisture content and ash values of herbal crude drug and formulation batches (pre and post stability) were determined, Moisture (loss on drying) is one of the major factors responsible for the deterioration of the drugs and formulations [8]. Low moisture content is always desirable for higher stability of herbal drugs (Table 2). Moisture contents of the individual Herbal drug were found in the range 1.39%-2.52%, w/w and different Formulation batches F1, F2, F3, F4 pre and post stability (Table 3) in the range of 1.44–2.48% w/w, all the values were less that 5%, were formulation F1, F2, F3 shows change in moisture content and F4 shows 1.44%-1.57% w/w moisture shows better stability, and high ash value is indicative of contamination, substitution, adulteration, or carelessness in preparing the drug or drug combinations for marketing [9]. All the individual drugs and different Formulation batches F1, F2, F3, F4 pre and post stability were determined. Ash values of the individual Herbal drug found to be total ash value in the range 4.53%-10.82% w/w, water-soluble ash in range of 2.43%-3.24% w/w and acid-insoluble ash in the range of 1.32%–2.46% w/w and ash values of different formulation batches F1, F2, F3, F4 pre and post stability were found to be, total ash value in the range of 9.32%-8.25% w/w, water soluble ash in the range of 2.98%-4.45% w/w, acid insoluble ash in the range of 0.9%-2.1% w/w, These values were found to be reasonably low indicating low contamination (Table 4). Moisture and ash values of the formulations matches with the average total ash values of the individual drugs [10]. Were formulation F1, F2, F3 shows change in values and F4 shows total ash 9.1-9.3, water soluble ash 4.43-4.45 and acid insoluble 1.9-2.0 having better stability.
pH and viscocity: pH and viscocity of the formulated instant soup powder was determined and found to be respectively.
Sensory analysis: Sensory evaluation is an essential concept in food product development as it reduces the risk of product failure and links the consumer perception about quality of food. Even though formulated food products are nutritious, without taste and odour the product cannot reach market in successfully. The results of sensory evaluation different formulation batches F1, F2, F3, F4 are illustrated in the Table 6. The data reveals that formulation F4 was found to be highly acceptability with significant difference when compared to other formulation F4 having better taste, odour, consistency and colour of soup [11-15]. From this study we finalize the F4 instant soup powder formulation as optimized formulation.
| Sr. no. | Name of drug powder and formulation | % LOD Mean (n=3) ± SD (% w/w) | Total ash Mean (n=3) ± SD (% w/w) | Water soluble ash Mean (n=3) ± SD (% w/w) | Acid insoluble ash Mean (n=3) ± SD (% w/w) |
|---|---|---|---|---|---|
| 1 | Jamun | 1.50 ± 0.17 | 4.53 ± 0.12 | 2.76 ± 0.35 | 1.72 ± 0.02 |
| 2 | Fenugreek | 2.52 ± 0.04 | 9.68 ± 0.24 | 2.43 ± 0.03 | 1.86 ± 0.02 |
| 3 | Gurmar | 1.39 ± 0.24 | 10.82 ± 0.14 | 3.24 ± 0.06 | 2.46 ± 0.02 |
Table 2: % LOD, total ash value water soluble ash acid insoluble ash for antidiabetic.
| Formulations | Pre stability | Post stability | ||
|---|---|---|---|---|
| % LOD Mean ± SD (% w/w) |
Total ash value Mean ± SD (% w/w) |
% LOD Mean ± SD (% w/w) |
Total ash value Mean ± SD (% w/w) |
|
| F1 | 2.48 ± 0.015 | 8.25 ± 0.065 | 3.13 ± 0.081 | 8.12 ± 0.095 |
| F2 | 2.38 ± 0.055 | 9.35 ± 0.045 | 3.19 ± 0.034 | 9.11 ± 0.078 |
| F3 | 1.78 ± 0.035 | 8.59 ± 0.11 | 2.27 ± 0.045 | 8.43 ± 0.065 |
| F4 | 1.44 ± 0.040 | 9.32 ± 0.075 | 1.57 ± 0.057 | 9.11 ± 0.076 |
Table 3: % LOD and total ash value for instant soup powder formulations.
| Formulations | Pre stability | Post stability (After 1 month) | ||
|---|---|---|---|---|
| Water soluble ash Mean ± SD (% w/w) | Acid insoluble ash Mean ± SD (% w/w) | Water soluble ash Mean ± SD (% w/w) | Acid insoluble ash Mean ± SD (% w/w) | |
| F1 | 4.10 ± 0.11 | 1.4 ± 0.043 | 3.87 ± 0.43 | 0.98 ± 0.32 |
| F2 | 3.65 ± 0.17 | 1.97 ± 0.43 | 3.28 ± 0.12 | 1.45 ± 0.11 |
| F3 | 3.15 ± 0.11 | 1.81 ± 0.21 | 2.98 ± 0.54 | 1.45 ± 0.098 |
| F4 | 4.45 ± 0.34 | 2.0 ± 0.32 | 4.43 ± 0.01 | 1.99 ± 0.087 |
Table 4: water soluble and acid insoluble ash values for instant soup powder formulations.
| Formulations | Pre stability | Post stability (After 1 month) | ||
|---|---|---|---|---|
| Viscosity (cPs) Mean ± SD |
pH Mean ± SD |
Viscosity (cPs) Mean ± SD |
pH Mean ± SD |
|
| F1 | 711 ± 3.51 | 4.53 ± 0.010 | 789 ± 4.5 | 4.19 ± 0.01 |
| F2 | 792 ± 4.58 | 4.43 ± 1.025 | 822 ± 2.08 | 5.72 ± 0.01 |
| F3 | 738 ± 5.10 | 4.16 ± 0.20 | 710 ± 3.512 | 4.97 ± 0.02 |
| F4 | 607 ± 4.93 | 4.13 ± 0.020 | 615 ± 2.00 | 4.26 ± 0.02 |
Table 5: pH and viscosity of instant soup powder formulation.
| Parameter | Very poor | Poor | Good | Very good | Excellence | |
|---|---|---|---|---|---|---|
| F1 | Odour | 10 | 16 | - | - | - |
| Taste | 12 | 7 | 7 | - | - | |
| Consistancy | 10 | 8 | 7 | 2 | - | |
| Color | 15 | 6 | 6 | - | - | |
| F2 | Odour | 9 | 11 | 7 | - | - |
| Taste | 10 | 15 | 2 | - | - | |
| Consistancy | 8 | 7 | 7 | 5 | - | |
| Color | 15 | 6 | 3 | 3 | - | |
| F3 | Odour | 3 | 13 | 8 | 3 | - |
| Taste | 2 | 9 | 10 | 6 | - | |
| Consistancy | - | 5 | 14 | 8 | 2 | |
| Color | - | 7 | 13 | 7 | - | |
| F4 | Odour | - | 2 | 8 | 15 | 2 |
| Taste | - | 1 | 10 | 13 | 3 | |
| Consistancy | - | 2 | 9 | 10 | 6 | |
| Color | - | 3 | 7 | 13 | 4 |
Table 6: Sensory analysis.
Estimation of nutritional values
Formulated instant soup powder was prepared using antidiabetic herb’s, spices, flavor and finger millet flour for thickening of soup and optimized formulation subjected for nutritional analysis and it was found to highly acceptable. The nutrient analysis of the formulation is represented in the Table 8 [16]. Protein content was found to be 10.55 gm/100 gm shows high in protein which required for diabetic patient, carbohydrates was found to 64.97 gm/100 gm which was in the safety range required for diabetic patient, total fat 1.06 gm/100 gm shows low total fat essential for diabetic patient, total fat 1.06 gm/100 gm shows low total fat essential for diabetic patient, energy value (calories) was found to 311.62 Kcal/100 gm shows acceptable calorie value [17]. The above study shows prepared formulation is in superior and safe nutritional content (Table 7).
| Sr. no. | Heavy metal | Result (mg/kg) |
|---|---|---|
| 1 | Lead (Pb ) | Absent |
| 2 | Arsenic (As) | Absent |
| 3 | Cadmium (Cd) | Absent |
| 4 | Mercury (Hg ) | Absent |
Table 7: Heavy metal.
Heavy metal content
Heavy metals are harmful and become toxic for health if they are taken above the limit of daily allowance recommended. In this study, heavy metals were not found in this antidiabetic nutraceutical formulation which shows formulation is safe (Table 8).
| Sr. no. | Nutritional value (per 100 gm) | |
|---|---|---|
| 1 | Energy value (Calories) | 311.62 Kcal/100 gm |
| 2 | Carbohydrates | 64.97 gm/100 gm |
| 3 | Total fat | 1.06 gm/100 gm |
| 4 | Protein | 10.55 gm/100 gm |
Table 8: Nutritional values.
Antimicrobial assesment
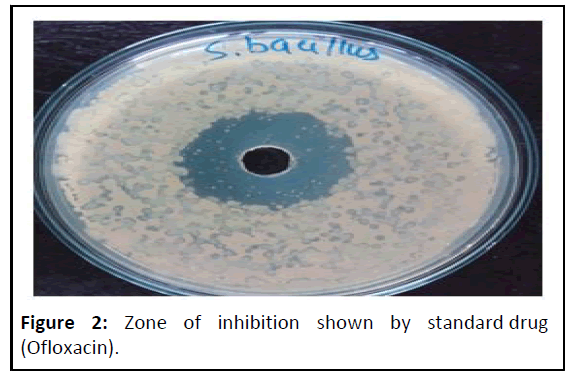
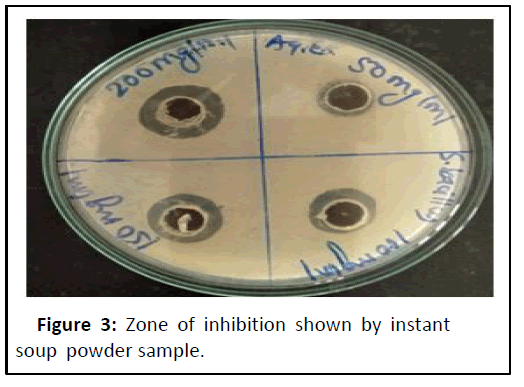
The standard drug ofloxacin and extract of Instant soup powder sample subjected for antimicrobial assessment for checking susaptability against S. bacillus. Were different concentration of sample extract shows zone of inhibition as follows: 50 mg/ml shows 10.50 mm, 100 mg/ml shows 12.50 mm, 150 mg/ml shows 13.50 mm, 200 mg/ml shows 16.00 mm against standard ofloxacin (Figures 2 and 3) were not superiar than std. but good susaptability shown by sample againt S. bacillus (Table 9).
| Strain used: S. bacillus zone of inhibition in mm | Standard drug (Ofloxacin) 10 mcg/ml | ||||
|---|---|---|---|---|---|
| Concentration | 50 mg/ml | 100 mg/ml | 150 mg/ml | 200 mg/ml | |
| Sample | 10.50 ± 0.577 | 12.50 ± 0.577 | 13.50 ± 0.577 | 16.00 ± 0.816 | 31.25 ± 0.957 |
Tabel 9: Zone of inhibition shown by standard and sample.
Antioxidant activity by DPPH assay
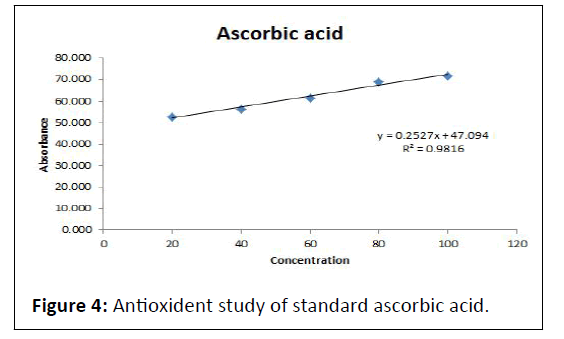
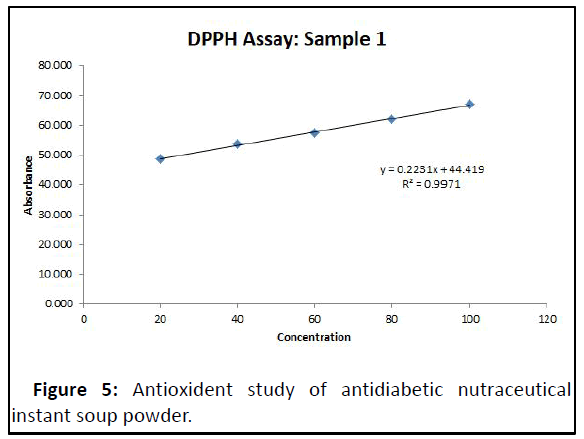
Antioxident activity of standard ascorbic acid and test sampleantioxident study of antidiabetic nutraceutical Instant soup powder the sample shows % inhibition for distinct concentration in range of 48.798 to 66.996% which was within range (Table 10 and Figure 4, Table 11 and Figure 5).
| Concentration in mg/ml | % Inhibition |
|---|---|
| 20 | 52.741 |
| 40 | 56.36 |
| 60 | 61.513 |
| 80 | 68.969 |
| 100 | 71.711 |
| IC50=11.54 | |
Table 10: % Inhibition of standard ascorbic acid in DPPH assay.
| Concentration in mg/ml | % Inhibition |
|---|---|
| 20 | 48.794 |
| 40 | 53.838 |
| 60 | 57.346 |
| 80 | 62.061 |
| 100 | 66.996 |
| IC50=25.06 | |
Tabel 11: % Inhibition of sample in HPPH assay.
Alpha amylase inhibition assay
Alpha amylase is an enzyme linked to diabetes is in agreement with earlier reports that showed that plant phytochemicals are mild inhibitors of alpha-amylase. A property that confers advantage over synthetic drugs such as acarbose; use by diabetics in the management of postprandial blood glucose, which strongly inhibit alpha-amylase stronger. Were standrd acarbose, sample 1 (Antidiabetic nutraceutical instant soup powder formulation), sample 2 (marketed) subjected to check alpha-amylase inhibition activity were standard shows % inhibition for 10 to 50 (μg/ml) in range of 47.66%-69.93%, sample 1 antidiabetic nutraceutical formulation (instant soup powder) shows % inhibition for 10 to 50 (μg/ml) in range of 45.68 to 56.58% and sample 2 marketed nutraceutical formulation shows 30.85% to 53% inhibition [18,19]. Were the above study shows formulated sample 1 antidiabetic nutraceutical formulation (instant soup powder) shows greater % inhibition than sample 2 marketed antidiabetic nutraceutical formulation and conform that the formulated antidiabetic nutraceutical formulation having antidiabetic activity (Tables 12-17) [20].
| Reading 1 | Reading 2 | Reading 3 | |||
|---|---|---|---|---|---|
| Conc. (µg/ml) | % Inhibition | Conc. (µg/ml) | % Inhibition | Conc. (µg/ml) | % Inhibition |
| 10 | 46.99 | 10 | 47.44 | 10 | 47.66 |
| 20 | 52 | 20 | 52.56 | 20 | 52.78 |
| 30 | 59.13 | 30 | 60.02 | 30 | 59.69 |
| 40 | 63.25 | 40 | 64.37 | 40 | 62.14 |
| 50 | 69.71 | 50 | 70.04 | 50 | 69.93 |
Table 12: % Inhibition of alpha amylase for standard acarbose.
| No. | IC50 (µg/ml) |
|---|---|
| Reading 1 | 15.5 |
| Reading 2 | 14.4 |
| Reading 3 | 14.3 |
| Mean ± SD | 14.73 ± 0.665 |
Table 13: IC50 for standard acarbose.
| Reading 1 | Reading 2 | Reading 3 | |||
|---|---|---|---|---|---|
| Conc. (µg/ml) | % Inhibition | Conc. (µg/ml) | % Inhibition | Conc. (µg/ml) | % Inhibition |
| 10 | 45.25 | 10 | 44.74 | 10 | 45.68 |
| 20 | 47.35 | 20 | 48.32 | 20 | 47.64 |
| 30 | 51.24 | 30 | 52.31 | 30 | 51.36 |
| 40 | 54.23 | 40 | 54.98 | 40 | 54.28 |
| 50 | 56.25 | 50 | 56.41 | 50 | 56.89 |
Table 14: % Inhibition of alpha amylase for standard acarbose for test sample 1 (Antidiabetic nutraceutical instant soup powder).
| S. no. | IC50 (µg/ml) |
|---|---|
| Reading 1 | 27.08 |
| Reading 2 | 25.5 |
| Reading 3 | 26.03 |
| Mean ± SD | 26.21 ± 0.805 |
Table 15: IC50 for test sample 1 (Antidiabetic nutraceutical instant soup powder).
| Reading 1 | Reading 2 | Reading 3 | |||
|---|---|---|---|---|---|
| Conc. (µg/ml) | % Inhibition | Conc. (µg/ml) | % Inhibition | Conc. (µg/ml) | % Inhibition |
| 10 | 31.07 | 10 | 32.85 | 10 | 30.85 |
| 20 | 35.3 | 20 | 35.63 | 20 | 36.75 |
| 30 | 43.1 | 30 | 43.43 | 30 | 43.76 |
| 40 | 48 | 40 | 48.55 | 40 | 48.78 |
| 50 | 55.12 | 50 | 56.57 | 50 | 53.23 |
Table 16: % Inhibition of alpha amylase for test sample 2 (marketed).
| S. No. | IC50 (µg/ml) |
|---|---|
| Reading 1 | 22.56 |
| Reading 2 | 21.72 |
| Reading 3 | 20.4 |
| Mean ± SD | 21.56 ± 1.084 |
Table 17: IC50 for test sample 2 (marketed).
Conclusion
Safe and effective antidiabetic nutraceutical formulation of combined antidiabetic herb’s and spices, flavor and thickners in the form of instant soup powder was successfully developed, the present investigation revealed that on the basic of sensory evaluation and intial accelerated stability study F4 formulation found to be superior than rest with least moisture content, and taken for further analysis and it shows absence of trace metals like arsenic, cadmium, lead and mercury, it is important to note that this soup is high in protein ash carbohydrate and low fat, high energy value of the soup powder which make it appropriate choice for fulfillment of nutritional demand. Further study for antioxident and antimicrobial. Shows the product is high in antioxident and antimicrobial. These studies for alpha-amylase inhibition assay for in vitro antidiabetic activity carried out and it was found that the formulated antidiabetic nutraceutical formulation (instant soup powder) is superior than the marketed nutraceutical formulation.
Throughout the experimental work experiments carried out under standard and safety measures. The use of conventional treatment of diabetes like insulin and drug like medformin etc. may have side effect in long run use. Now a day nutraceutical formulations is in trend to control various diseases. Nutraceuticals play important role in controlling diabetes.
Nutraceutical herb’s like jamun seed, fenugreek seed, gurmar leaves play vital role in controlling diabetes by make use of this antidiabetic herb’s antidiabetic nutraceutical formulation were formulated in the form of Instant soup powder formulation by making use of spices like garlic, black paper, curry leaves salt, tevia for sweetening, tomato for flavor, fingermillet for thickening and oyster mashrrom for thickening and flavor all the ingridients are collected from local market having good quality.
The formulated antidiabetic nutraceutical formulation were evaluated for sensory evaluation intitial accelerated stability study, pH, viscocity, moisture, ash values, micromeritics properties, nutritional values, heavy metal content, antioxident and antimicrobial properties and in vitro alpha amylase inhibition assay. Were formulation showed satisfactory results. The stability studies were carried on the basis of ICH guideline for 90 days.
References
- Gupta R, Bajpai G, Johri S, Saxena M (2008) An overview of Indian novel traditional medicinal plants with antidiabetic potentials. Afr J Tradit Complement Altern Med 5:51‑72
[Google Scholar] [PubMed]
- Paolisso G, Amero D, Di Maro G, Galzerano D, Tesauro P, et al. (1993) Evidence for a relationship between free radicals and insulin action in elders. Metabolism 42:659‑663
[Crossref] [Google Scholar] [PubMed]
- Tiwari K, Rao M (2002) Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. J Curr Sci 83:30‑38
- Baldi A, Choudhary N, Kumar S (2013) Nutraceuticals as therapeutic agents for holistic treatment of Diabetes. Int J Green Pharm 7:278-287
- Mahdi A, Chandra A, Singh K, Shukla S, Mishra C, et al. (2003) Effect of herbal hypoglycemic agents on oxidative stress and antioxidant status in diabetic rats. Indian J Clin Biochem 18:8‑15
[Crossref] [Google Scholar] [PubMed]
- Karawya M (1980) Mucilagenous contents of certain Egyption plants. J Planta Med 38:73–78
- Sudarshan M, Visalachi A (2017) Development and formulation of instant soup mix from sprouted horse gram and radish leaves. Int J Home Sci 3:346-349
- Farzana T, Mohajan S (2017) Formulation and nutritional evaluation of a healthy vegetable soup powder supplemented with soy flour, mushroom, and moringa leaf. Food Sci Nutr 5:911–920
[Crossref] [Google Scholar] [PubMed]
- Bauer AW, Kirby WMM, Sherris JC, Turck M (1996) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493
[Google Scholar] [PubMed]
- Gulcin I, Elias R, Gepdiremen A, Boyer L (2006) Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 223:759-767
- Iniyan G Tamil, Dineshkumar B, Nandhakumar M, Senthilkumar M, Mitra A (2010) In vitro study on α-amylase inhibitory activity of an Indian medicinal plant Phyllanthus amarus. Indian J Pharmacol 42:280–282
[Crossref] [Google Scholar] [PubMed]
- Abheyasinghe C, Illeperuma C (2006) Formulation, development of MSG (Monosodium Glutamate) free instant vegitable soup mix. J Natl Sci Found 34:91-95
- Niththiya N, Vasantharuba S (2016) formulation of instant soup mix powder using uncooked palmyrah (Borassus flabellifer) tuber flour and locally available vegetables. J Food Nutr 198-202
- Bauer AW, Kirby WMM, Sherris JC, Turck M (1996) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493
- Gulcin I, Elias R, Gepdiremen A, Boyer L (2006) Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 223:759-767
- Jain R, Jain SK (2011) Total phenolic contents and antioxidant activities of some selected anti-cancer medicinal plants from Chhattisgarh state, India. Apoptosis 12:1-14
- Das G, Patra JK, Debnath T, Ansari A, Shin HS (2019) Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PloS One 14:e0220950
[Crossref] [Google Scholar] [PubMed]
- Iqbal R, Liaqat A, Saeed F, Khaliq A, Jahangir Chughtai MF, et al. (2021) Zogale (Moringaolifera) as a functional ingredient: A review on its nutraceutical properties and food applications. Int J Food Prop 24:1202-1213
- Shimizu M, Kubota M, Tanaka T, Moriwaki H (2012) Nutraceutical approach for preventing obesity related colorectal and liver carcinogenesis. Int J Mol Sci 13:579-595
[Crossref] [Google Scholar] [PubMed]
- Durazzo A, Lucarini M, Novellino E, Souto EB, Daliu P, et al. (2018) Abelmoschus esculentus (L.): Bioactive components’ beneficial properties—focused on antidiabetic role—for sustainable health applications. Molecules 24:38
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences