Effect of Betalain-Rich Concentrate on Exercise Performance in Young, Healthy, Sedentary Individuals: A Double-Blind, Crossover, Placebo-Controlled Pilot Study
Tania Reyes-Izquierdo, Ruby Argumedo, Melissa MC Afram, Boris Nemzer and Zbigniew Pietrzkowski
Tania Reyes-Izquierdo*, Ruby Argumedo,Melissa MC Afram,Boris Nemzer and Zbigniew Pietrzkowski
Bioresearch Laboratory, VDF Futureceuticals Inc, Irwin, California, USA
- *Corresponding Author:
- Tania Reyes-Izquierdo
Bioresearch Laboratory, VDF Futureceuticals
Inc 23 Peters Canyon Rd, Irvine, CA, 92606
USA
Tel: +1 949 502 4496
Fax: +1 949 502 4987
E-mail: treyes@futureceuticals.com
Received date: November 02, 2017; Accepted date: December 11, 2017; Published date: December 18, 2017
Citation: Izquierdo TR, Argumedo R, Afram MMC, Nemzer B, Pietrzkowski Z. (2017) Effect of Betalain-Rich Concentrate on Exercise Performance in Young, Healthy, Sedentary Individuals: A Double-Blind, Crossover, Placebo-Controlled Pilot Study. J Nutraceuticals Food Sci. Vol.2 No.3:17
Abstract
Background: Betalain-rich concentrate (BRC) improved the performance of competitive male runners and triathletes in randomized and controlled studies. In this pilot study, we tested the effect of a single dose of BRC in sedentary individuals on exercise performance. Methods and findings: Eleven non-athletic sedentary but otherwise healthy subjects completed this double-blind crossover (placebo and BRC) randomized study. 150 minutes after supplementation, each subject initiated exercise on stationary steppers and were instructed to “try your best” for 30 minutes. Results indicate that subjects had a significantly higher number of steps after a single dose of BRC (1699.73 ± 400.89 steps) when compared to placebo (1496.45 ± 357.64), (P=0.0186). The total count of burned calories was higher in the BRC group when compared to the placebo group (p=0.014). Blood lactate was higher in both experimental groups after 30 minutes of exercise, with no significant differences between the two groups. Conclusion: Our results suggest that BRC improves exercise performance in sedentary individuals.
Keywords
Betalain rich concentrate; Exercise performance; Betalains
Introduction
Betalains are water-soluble, nitrogen-containing pigments that possess high bioactivity [1-3]. Betalains have particularly potent antioxidant capacity and protect cell membranes from lipid peroxidation [4]. Beetroot-derived betalains also protect cells from nitrosative stress, inhibiting peroxynitrite-mediated tyrosine nitration [5]. Additionally, these pigments exhibit free radical scavenging activity [6]. It was reported that the effectiveness of the betalains, betanin and indicaxanthin in scavenging hypochlorous acid, which is the most potent oxidant produced by human neutrophils [7]. Our previous research showed that betalains significantly reduced blood levels of proinflammatory cytokines [8].
Intense exercise induces muscle microtrauma and elicits an inflammatory response that interferes with performance and increases muscle and joint pain [9]. Recent work has shown that athletes who consumed BRC for six days experienced substantially increased performance and benefited during the recovery period after exercise [1,2]. Since potentially even greater exercise-induced muscle damage can occur in people unaccustomed to exercise, we examined the effects of betalainrich concentrate (BRC), a proprietary concentrate having no less than 25% betalains and is substantially free of sugars and nitrateson performance and biochemical markers in sedentary volunteers prior to and after exercise [8,10,11]. Specifically, we quantified amount of exercise performed, caloric utilization and changes in various biochemical parameters. Our results suggest BRC supplementation improves exercise performance in sedentary individuals.
Materials and Methods
Study materials
Beet-root concentrate (BRC), a material derived from red beet (Beta vulgaris L.) substantially depleted of sugars and enriched to a total betalains content of at least 25%, was provided by FutureCeuticals, Inc. (Momence IL, USA). The relative composition of BRC has been previously reported by Nemzer et al. [12]. BRC contains levels of betanin (23.8%), isobetanin (30.3%), 17-decarboxy-betanin (3.6%), 17-decarboxy-isobetanin (4.7%), neobetanin (16.8%). The placebo was an empty gelatin capsule (Capsuline®, Pompano Beach, FL, USA).
Clinical study
This double-blind, placebo-controlled clinical study was conducted according to guidelines laid out in the Declaration of Helsinki. All procedures involving human subjects were approved by the Institutional Review Board (Comité de Ética en Investigación Biomédica para el Desarrollo de Fármacos, S.A. de C.V., Av. Sebastian Bach No. 5257, Col. La Estancia, C.P. 45030, Zapopan, JAL, Mexico) (IRB: FCENCI- 16-06-KNN). All participants provided written, informed consent before any experimental procedure was performed.
Exercise intensity and frequency levels were classified as “sedentary” (no exercise beyond basic activities of daily living), “light” (occasional dog walks, strolls through the park, etc.), “moderate” (occasional workouts on less than 2 days per week), or “vigorous” (frequent workouts, physical training, or hiking). All recruited participants were sedentary and engaged in less than 20 minutes of structured exercise per week at the time of enrolment.
Exclusion criteria included diagnosis of diabetes mellitus, allergies to dietary products, history of strenuous exercise, use of anti-inflammatory drugs, analgesics, statins, diabetic drugs, anti-allergy medicines or multivitamins, and use of dietary supplements of any kind within 15 days of the start of the study. Three subjects were excluded from the study for lack of compliance. Subjects had no food allergies, rhinitis, influenza, or other acute infections. Subjects were advised to abstain from vigorous exercise during the study.
Blood collection
Participants were instructed to fast for 12 h prior to the initial blood draw. Blood samples were collected at baseline (T0), prior to taking study treatment (BRC or placebo), prior to exercise (T150), after exercise(T180) and after a 30-min resting period (T210). In total, 450 μL of blood were collected by finger puncture for immediate tests and for plasma separation. For blood gas analysis, one hundred microliters (100 μL) were collected in Safe- T-Fill® Capillary Lithium Heparin Blood collection tubes (Ram Scientific Inc. Yonkers, NY), as well as 250 μL of blood for heparin plasma and one hundred microliters (100 μL) of blood were collected in Safe-T-Fill® Capillary EDTA Blood collection tubes (Ram Scientific Inc. Yonkers, NY) for EDTA plasma.
Exercise protocol
Testing was performed using either placebo or BRC on two separate days. A 10-day recovery period was given between Day 1 and Day 2. On Day 1 of the study, subjects were given capsules containing either 50 mg of BRC or placebo in a randomized manner at T0 following baseline blood draws. Participants were also given 350 ml of water, and advised to reserve a portion to drink with provided snacks. After 30 minutes following capsule intake, (T30), subjects were given two Smucker’s Uncrustables® sandwiches (The JM Smucker Co, Orrville, OH, USA). Each standardized snack contained 210 kcal with 28 g of total carbohydrates, 9 g of fat and 6 g of protein. Finger blood was subsequently collected 2 h (T150) (120 minutes following ingestion of the snacks). Afterwards, subjects were prompted to exercise and “try their best” for 30 min. In order to provide ample time for recuperation and also to reduce the chance of physical conditioning, a ten-day recovery period was allocated between Day 1 and Day 2 of the protocol. On Day 2, subjects were administered the remaining (crossover) supplementation test capsules. Subjects and staff were blinded to the content of the capsules throughout the study.
The exercise steppers used were from Sunny Health and Fitness Mini Stepper with Resistance Bands (City of Industry, California, USA). The steppers were set to medium resistance and subjects were encouraged to exercise at maximum capacity (i.e. to “try their best”) for 30 min. Subjects were instructed to take full steps during exercise. The number of steps completed, and total calories burned were recorded by the ministeppers. Blood oxygen saturation was measured using a portable finger pulse oximeter OX SpO2 Fingertip rate were recorded prior to exercise (T150) and every 10 min during exercise. Immediately after exercise, blood was collected (T180) as described. Weight and body water were determined before and after exercise using a Weight Watchers® glass body analysis scale model WW52Y by Conair (East Windsor, New Jersey, USA). Blood oxygen saturation, heart rate, body weight and, body water, were measured again after a 30-min resting period.
Glucose and lactate measurement
Glucose and lactate measurement were taken at every time point previously indicated. For the blood glucose measurement an Accu-chek® Compact plus (Roche, Basel Switzerland) was used. A small amount of pressure was directed toward the puncture site to extract a few droplets for the glucose meter reading pad. Once the meter gave a glucose reading, a few more droplets of blood were taken to measure the blood lactate using a lactate Scout+ meter (EKF Diagnostics, Leipzig, Germany).
Clinical blood analysis
For the determination of blood gases, finger blood samples were analyzed with a handheld clinical blood gas analyzer (i-STAT® Point of Care, Abbot Laboratories, NJ, USA). After fresh finger blood was collected into a lithium heparin tube (RAM Scientific, Yonkers, NY, USA), 100 μL of blood were added to CG8+ cartridges (Abbot Laboratories, NJ, USA) for pH, partial pressure of carbon dioxide (PCO2), partial pressure of oxygen (PO2), total carbon dioxide levels (TCO2), bicarbonate (HCO3), base excess (BEecf), oxygen saturation (sO2), sodium (Na), potassium (K), ionized calcium (iCa), glucose (Glu), haematocrit (Hct) and hemoglobin (Hb). This procedure was followed for every finger blood collection time point (T0, T150, T180 and T210). Results for every time point’s parameters were normalized to their T0 values and results are reported as percentage change over baseline.
Statistical analysis
Lactate, hemoglobin and glucose levels as well as partial pressure of oxygen were normalized using time zero as the baseline. All statistical evaluations were performed using the commercially available GraphPad® statistical software (Graphpad Software Inc., La Jolla, CA, USA). The data expressed as mean ± SEM were first tested for normality (Shapiro-Wilk normality test). Since parametric assumptions were not fully met, we used nonparametric analyses of variance (Friedman’s test) followed by Wilcoxon rank-sum tests. Differences were considered significant at the p< 0.05 level.
Results
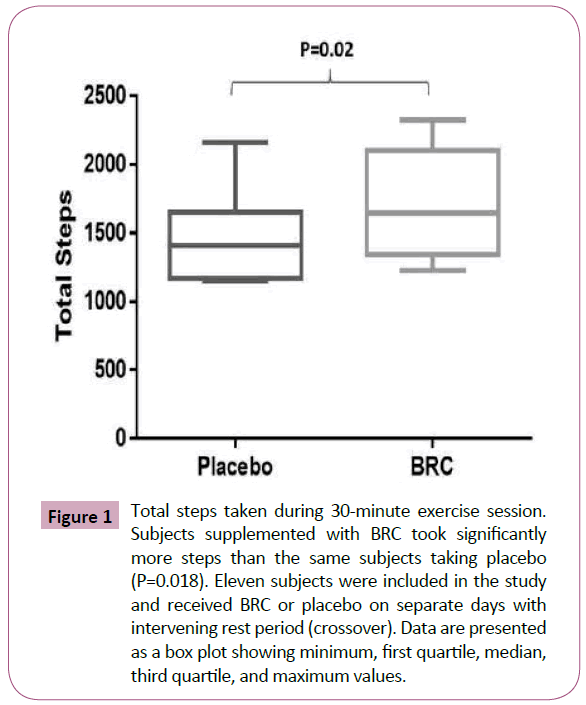
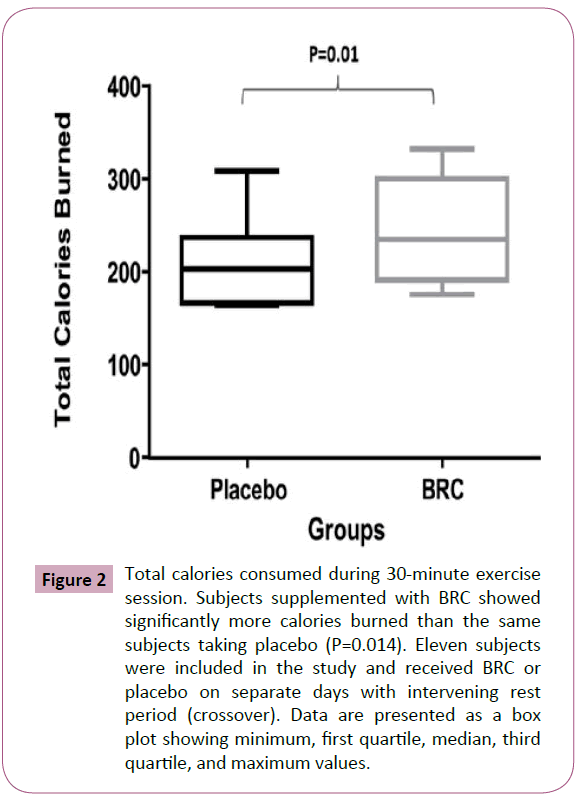
Eleven subjects completed this double blind, placebo-controlled, crossover clinical study. Eleven subjects (six female and five male) completed the protocol by randomly taking both the placebo and BRC on separate days. The mean age of the subjects was 33.4 ± 6.6 years and mean BMI was 26.7 ± 6.83 kg/m². No significant differences in blood oxygen saturation or heart rate as determined by capillary blood were observed between BRC and placebo users during exercise. When subjects were supplemented with BRC, they took a significantly greater number of steps than they did when treated with placebo (1699.73 ± 400.89 steps vs placebo: 1496.45 ± 357.64, P=0.019) (Figure 1). BRC-supplemented subjects burned more calories than did subjects treated with placebo (242.4 ± 57.23 calories vs placebo: 213.55 ± 51.11 calories, P=0.014) (Figure 2).
Figure 1: Total steps taken during 30-minute exercise session. Subjects supplemented with BRC took significantly more steps than the same subjects taking placebo (P=0.018). Eleven subjects were included in the study and received BRC or placebo on separate days with intervening rest period (crossover). Data are presented as a box plot showing minimum, first quartile, median, third quartile, and maximum values.
Figure 2: Total calories consumed during 30-minute exercise session. Subjects supplemented with BRC showed significantly more calories burned than the same subjects taking placebo (P=0.014). Eleven subjects were included in the study and received BRC or placebo on separate days with intervening rest period (crossover). Data are presented as a box plot showing minimum, first quartile, median, third quartile, and maximum values.
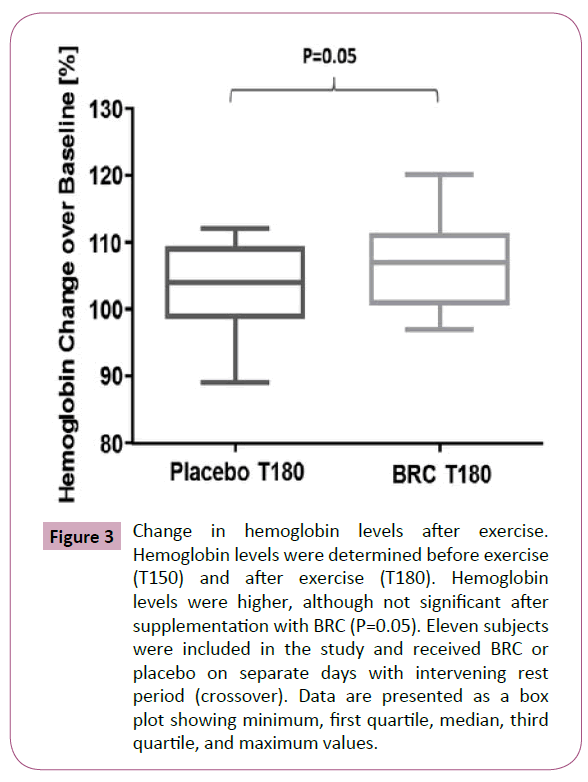
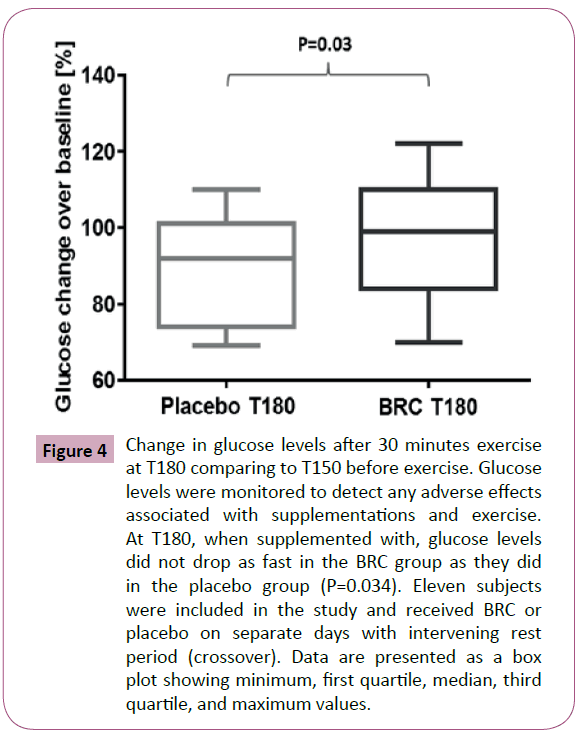
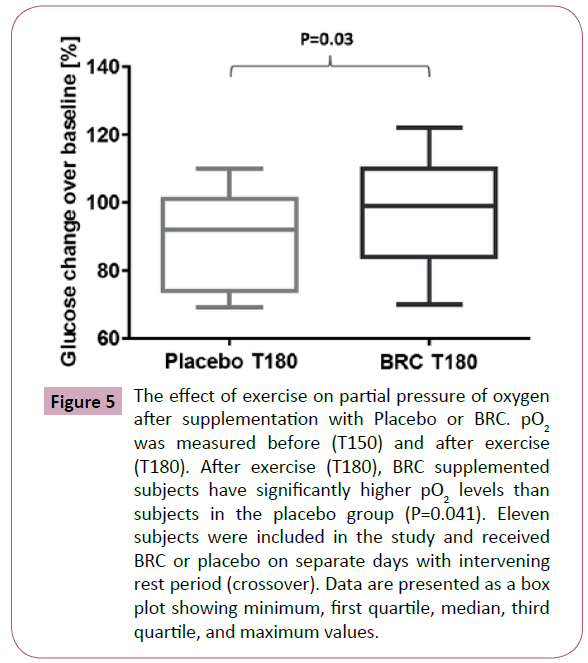
Even though lactate increased to 525% after exercise in the placebo group and 566% in the BRC supplemented group, no statistical differences were observed between both supplementations (data not shown). Also, no significant differences were observed on hemoglobin levels (P=0.05), as can be observed in Figure 3. Glucose levels were also monitored during the study to detect any adverse effects associated with the supplementations or with exercise. Blood glucose levels were not statistically different between supplemented groups at T0 or T150; however, subjects supplemented with BRC had higher blood glucose levels than placebo users at T180, after completion of exercise (P=0.03) (Figure 4). No significant differences were observed while measuring partial pressure of oxygen at baseline, before exercise and after exercise (Data not shown) between both groups. However, subjects supplemented with BRC showed a significant increase in PO2 (P=0.04), when T150 was used as baseline over T180 just after completion of exercise (Figure 5).
Figure 3: Change in hemoglobin levels after exercise. Hemoglobin levels were determined before exercise (T150) and after exercise (T180). Hemoglobin levels were higher, although not significant after supplementation with BRC (P=0.05). Eleven subjects were included in the study and received BRC or placebo on separate days with intervening rest period (crossover). Data are presented as a box plot showing minimum, first quartile, median, third quartile, and maximum values.
Figure 4: Change in glucose levels after 30 minutes exercise at T180 comparing to T150 before exercise. Glucose levels were monitored to detect any adverse effects associated with supplementations and exercise. At T180, when supplemented with, glucose levels did not drop as fast in the BRC group as they did in the placebo group (P=0.034). Eleven subjects were included in the study and received BRC or placebo on separate days with intervening rest period (crossover). Data are presented as a box plot showing minimum, first quartile, median, third quartile, and maximum values.
Figure 5: The effect of exercise on partial pressure of oxygen after supplementation with Placebo or BRC. pO2 was measured before (T150) and after exercise (T180). After exercise (T180), BRC supplemented subjects have significantly higher pO2 levels than subjects in the placebo group (P=0.041). Eleven subjects were included in the study and received BRC or placebo on separate days with intervening rest period (crossover). Data are presented as a box plot showing minimum, first quartile, median, third quartile, and maximum values.
Discussion
The principal finding of this study is that a single 50 mg dose of BRC significantly improved exercise capacity in sedentary individuals. In this study, subjects were instructed to try their best during a 30-minute exercise period. Subjects who took BRC 2.5 hours prior to exercise were able to achieve more steps and burn more calories. Since this was a crossover study, performance was compared within subjects, i.e. subjects taking BRC outperformed their own results obtained when taking placebo. Previous studies have reported the benefits of BRC after a 6-day supplementation in male athletes and triathletes [1,2]. We now show for the first time that acute BRC supplementation results in detectable exercise benefits in non-athletes.
Glucose utilization does not appear to be higher with BRC supplementation despite increased caloric expenditures. Indeed, glucose levels increase similarly in both placebo and BRC groups after consuming a snack, yet glucose levels stayed higher in the BRC group throughout subsequent the exercise period. If BRC was achieving elevated glucose by producing a relative hyperglycemia directly, one would expect to see elevated glucose levels prior to exercise, which did not occur. This may indicate that BRC stimulates the utilization of fats, rather than sugars, in order to maintain sufficient energy for exercise performance by sedentary individuals. Interestingly, Van Hoorebeke et al showed a trend toward increased fat utilization and decreased glucose utilization with BRC supplementation in athletes who performed 30 minutes of submaximal exercise [1].
Despite expending more calories and achieving more steps, PO2 levels were significantly higher after 30 minutes of exercise comparing to T150 level (just before exercise) in subjects receiving BRC supplements acutely. This was perhaps due to a higher oxygen-carrying capacity of blood in the BRC group since BRC also increased hemoglobin levels. While we cannot directly extrapolate exercise capacity or efficiency from venous blood oxygen measurements without quantifying arterial oxygen levels and/or maximal oxygen consumption, the greater availability of oxygen may have contributed to increased calorie consumption observed in our research subjects. The mechanism for this increase remains to be elucidated.
Conclusion
As mentioned above, our results demonstrate that acute supplementation of BRC improves exercise performance and calorie utilization in sedentary individuals. This effect could also be attributed to the betalain content in BRC, since there were negligible levels of nitrates or sugars its composition. This is of significant interest due to the fact that most research on beetroot and beetroot products has been focused on nitrates and their function [13]. BRC seems to be working in a different manner yet has to date manifested similar or even superior effects on performance as compared to other high-nitrate red beet preparations [14]. The data herein reported supports and extends previous research showing that BRC had beneficial properties, enhancing exercise performance in athletes. Since our results show that subjects who exercised after a single BRC supplementation appear to utilize non-glucose energy sources, preferentially future work is planned to explore this possibility and to further investigate its biological mechanisms of action.
Funding
The present study was funded by Futureceuticals, Inc. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data.
Conflict of Interest
All authors declare that they have no conflict of interest.
Acknowledgments
We express our gratitude to John Hunter and Nagendra Rangavajla (FutureCeuticals) for their comments and suggestions in the preparation of this article. We would like to thank Michael Sapko for his help in editing the manuscript.
References
- Van Hoorebeke J, Trias C, Davis B, Lozada C, Casazza G (2016) Betalain-rich concentrate supplementation improves exercise performance in competitive runners. Sports 4: 40.
- Montenegro CF, Kwong DA, Minow ZA, Davis BA, Lozada CF, et al. (2017) Betalain-rich concentrate supplementation improves exercise performance and recovery in competitive triathletes. Appl Physiol Nutr Metab 42: 166-172.
- Tesoriere L, Allegra M, Butera D, Livrea MA (2004) Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am J Clin Nutr 80: 941-945.
- Clifford T, Howatson G, West DJ, Stevenson EJ (2015) The potential benefits of red beetroot supplementation in health and disease. Nutrients 7: 2801-2822.
- Sakihama Y, Maeda M, Hashimoto M, Tahara S, Hashidoko Y (2012) Beetroot betalain inhibits peroxynitrite-mediated tyrosine nitration and DNA strand cleavage. Free Radic Res 46: 93-99.
- Belhadj Slimen I, Najar T, Abderrabba M (2017) Chemical and antioxidant properties of betalains. J Agric Food Chem 65: 675-689.
- Allegra M, Tesoriere L, Livrea MA (2007) Betanin inhibits the myeloperoxidase/nitrite-induced oxidation of human low-density lipoproteins. Free Radic Res 41: 335-341.
- Pietrzkowski Z, Nemzer B, Spórna A, Stalica P, Tresher W, et al. (2010) Influence of betalain-rich extract on reduction of discomfort associated with osteoarthritis. New Med 1: 12-17.
- Chatzinikolaou A, Draganidis D, Avloniti A, Karipidis A, Jamurtas AZ, et al. (2014) The microcycle of inflammation and performance changes after a basketball match. J Sports Sci 32: 870-882.
- Howatson G, Van Someren KA (2008) The prevention and treatment of exercise-induced muscle damage. Sports Med 38: 483-503.
- Pietrzkowski Z, Argumedo R, Shu C, Nemzer B, Wybraniec S, et al. (2014) Betalain-rich red beet concentrate improves reduced knee discomfort and joint function: A double blind, placebo controlled pilot clinical study. Nutr Diet Sup 6: S59042.
- Nemzer B, Pietrzkowski Z, Spórna A, Stalica P, Thresher W, et al. (2011) Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem 127: 42-53.
- Jones AM (2014) Dietary nitrate supplementation and exercise performance. Sports Med 44 Suppl 1: S35-45.
- https://caroltorgan.com/athletic-performance-beetroot-juice-nitrates-spit/
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences