Determination of Caffeine Content in Several Over-the-Counter Energy Supplements
Richard W Dudley, Blain S Patrick, Daniel J Weiland and Sandra B Earle
Richard W Dudley*, Blain S Patrick, Daniel J Weiland and Sandra B Earle
College of Pharmacy, The University of Findlay, Ohio, USA
- *Corresponding Author:
- Richard W. Dudley
College of Pharmacy, The University
of Findlay, 1000 North Main Street
Findlay, Ohio 45840, USA
Tel: 419-434-5387
E-mail: dudley@findlay.edu
Received Date: September 01, 2017 Accepted Date: September 17, 2017 Published Date: September 23, 2017
Citation: Dudley RW, Patrick BS, Weiland DJ, Earle SB (2017) Determination of Caffeine Content in Several Over-the-Counter Energy Supplements. J Nutraceuticals Food Sci. Vol. 2 No. 3:11
Abstract
Background: Caffeine consumption continues to escalate and purchases of caffeine-containing beverages are increasing. The widespread availability of caffeinated beverages along with numerous caffeinated supplements creates concerns for significant adverse outcomes from overconsumption of caffeine. Despite labelling with caffeine content on packaging, over-the-counter (OTC) supplements are not required to validate actual caffeine content. When caffeine is derived from a natural source the amount of caffeine may be difficult to standardize and quantify. Therefore, there is a need to accurately measure the actual amount of caffeine content in OTC supplements available at local retailers.
Methods and Findings: A high pressure liquid chromatography method to determine caffeine concentrations was developed. The caffeine content in three OTC supplements (Hydroxy-Cut, Black Jax, and Swarm) were measured. A standard curve was created and utilized to determine caffeine concentrations using area under the curve data.
Results and Discussion: Of the three supplements tested a higher than stated caffeine concentration was routinely detected in Hydroxy-cut samples with an average concentration 20% above what is stated on the product labelling. Black Jax and Swarm were roughly at or below the ± 10% threshold required by the United States Pharmacopeia for stated content.
Conclusion: Stated caffeine content on OTC supplements described as being energy boosters or weight loss supplements may or may not be as labelled. Consumers and health care workers should be aware that caffeine content may vary from product to product especially if the source of caffeine is difficult to quantitate.
Keywords
Caffeine; Beverages; Supplements
Introduction
Caffeine, a methylxanthine (Figure 1), is ubiquitous in society and is perhaps the most widely consumed stimulant in the world. Beverages such as soda pop and coffee are popular and well known to contain caffeine. Many other products containing caffeine include sports and energy drinks, OTC headache medications and weight loss supplements. When ingested, caffeine produces numerous physiological and neurological effects including diuresis, central nervous system stimulation (alertness and arousal), and insomnia [1,2]. Additionally, caffeine ingestion can provoke increases in blood pressure and systemic vascular resistance [3]. Caffeine, at higher concentrations, also acts as an inhibitor of phosphodiesterase, the enzyme responsible for degrading cyclic nucleotides [1]. Prolonging the activity of intracellular cyclic adenosine monophosphate (cAMP) may enhance neurotransmitter and/or hormonal release adding to the varied effects of caffeine administration.
The physiological effects described for caffeine are dosedependent and increased caffeine intake is associated with severe adverse effects [4]. Despite the risk of adverse effects, caffeine continues to be used for its stimulant effects. Caffeine is also used for several clinical indications including the treatment of apnea in premature infants and neonates [5]. In this setting, caffeine acts as an antagonist of adenosine receptors to block the negative neuromodulatory effects of adenosine and stimulates respiratory neural output [6,7]. Caffeine is also used intravenously for spinal puncture headaches and has also been co-formulated with other analgesics (aspirin, acetaminophen, opioids) for the treatment of pain and migraine headaches [8,9]. When used under medical supervision, adverse effects of caffeine are less likely to occur. However, unsupervised consumption of caffeine in OTC products may produce significant adverse effects.
Inappropriate consumption of caffeine-containing beverages, in particular energy drinks, carries the risk of severe adverse effects. Caffeine consumption in the form of sports drinks has been linked to hypertension, CNS disorders, and fatalities [10-15]. Use of OTC caffeine supplements also poses a significant health risk. What may be more concerning is that the production of OTC caffeine supplements is highly unregulated. While the FDA oversees all prescription products, including those containing caffeine, the only required labelling for caffeine-containing dietary supplements is to list the total amount of caffeine per unit [16]. This may be difficult to quantitate precisely. Proprietary blends used in many supplements may have varying amounts of caffeine if using plant-based sources of caffeine such as guarana and kola nut as well as yerba mate [16]. Who then validates the caffeine content in dietary supplements? To add to the concern, the consumer of these products has no guidance on what these caffeine amounts actually mean. Should patients at risk from high concentrations of caffeine (e.g. atrial fibrillation, hypertension, arrhythmias) be given a maximum daily dose of caffeine to stay within? Unfortunately, there is no well-defined limit to daily caffeine intake. In fact, consumption of caffeinated coffee may offer protection from numerous comorbidities [17].
Given that caffeine overdose is a real concern and since oral dosage forms (capsule/tablet) allow for the rapid ingestion of copious quantities of caffeine in a brief period of time, we wanted to determine the accuracy of the caffeine content stated on various supplements. In order to address this concern, three different caffeine-containing supplements marketed as energy boosters or weight-loss supplements were purchased. A highpressure liquid chromatography (HPLC) method to separate and determine the caffeine concentration in each of the three products was developed and used for this study.
Materials and Methods
Caffeine (USP) was purchased from Sigma Aldrich (St. Louis, MO) and used as our standard. The mobile phase consisted of analytical grade acetonitrile (ACN) which was purchased from Fisher Scientific (Hanover Park, IL) and deionized water (18 Ω) which was readily available at our facility. Separation and analysis was conducted on a Hypersil ODS® reverse-phase analytical column (150 mm × 4.6 mm ID, 5 μm particle size). Hydroxy-Cut was purchased online from Amazon.com® while Swarm and Black Jax capsules were purchased from local gas stations. All other reagents used were of analytical grade.
Instruments and chromatographic conditions
An Agilent 1100 HPLC system consisting of a binary pump (model G1312A, serial number US72100921), an autosampler (G1313A, DE11114719), a diode array detector (G1315A, US82403082), a column thermostat (G1316A, DE23930617), and a degasser (G1322A, JP73015922) was used for chemical separation and analysis. Data was collected using Agilent ChemStation software. A Hypersil ODS® column (150 mm x 4.6 mm ID) was used in the analysis. The mobile phase consisted of deionized water and acetonitrile 40:60 (v/v). This mixture was isocratically pumped at a flow rate of 1 mL.min-1 for a 10-minute run with thermostat controlled temperature of 30°C. The detection wavelength for caffeine was set at 254 nm following an injection volume of 5 μL per sample. Under the described chromatographic conditions, the retention time of caffeine was 2.15 minutes.
Standard Curve
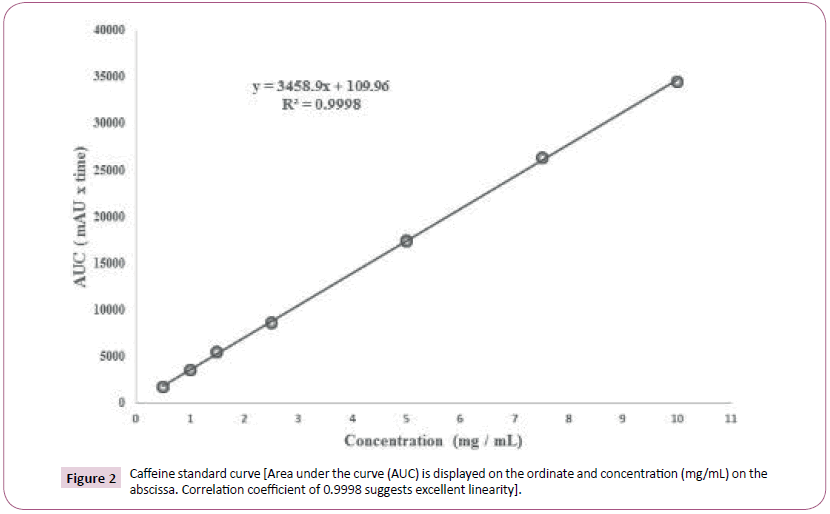
Caffeine (200 mg) was accurately weighed and diluted with hot water (80°C) to a concentration of 10 mg/mL in a volumetric flask. Subsequent dilutions (0.5 to 10 mg/mL) were prepared in water and a 5 μL sample of each dilution was injected (n = 5) using the aforementioned isocratic method. Quintuplicate samples were used to generate area under the curve (AUC) data for which linear regression analysis was performed to establish the concentration (mg/mL) versus AUC relationship in quantitative terms.
The relationship is expressed by the equation:
y = 3458.9x + 109.96
From above, ‘x’ represents the concentration of caffeine and y represents AUC for caffeine (dimensionless value). The correlation coefficient (R2) was 0.9998 gave excellent linearity. Concentrations of each supplement test sample were then calculated using the aforementioned equation also depicted in Figure 2.
Sample preparation (General procedure)
Caffeine is reported to be soluble in water (approximately 16 mg/ mL at room temperature), however increasing the temperature of water to 80°C increases caffeine solubility to approximately 200 mg/mL [18]. Therefore, all samples were solubilized and diluted in hot water (80°C) then filtered and placed in sample vials. For all solid dosage forms (Hydroxy-Cut, Swarm, and Black Jax), capsules were opened and placed into a 100 mL graduate cylinder followed by the addition of hot water to a final volume of 100 mL. This volume was transferred to a screw top bottle and vigorously shaken. Next, 2 mL of each sample were drawn, filtered, and transferred to a labelled vial and loaded onto the autosampler. Each sample (Hydroxy-Cut, Black Jax, and Swarm) was prepared in triplicate. Each major peak in the resulting sample chromatogram was compared to that of standards and subsequently identified as caffeine based on retention time.
Results
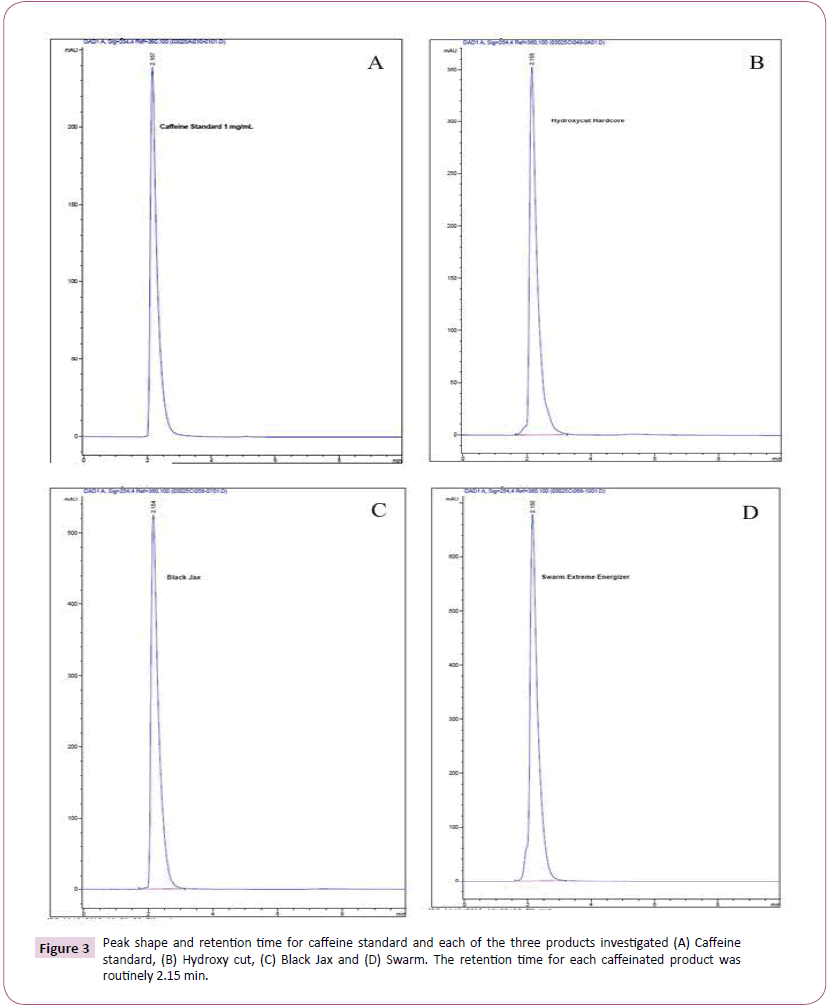
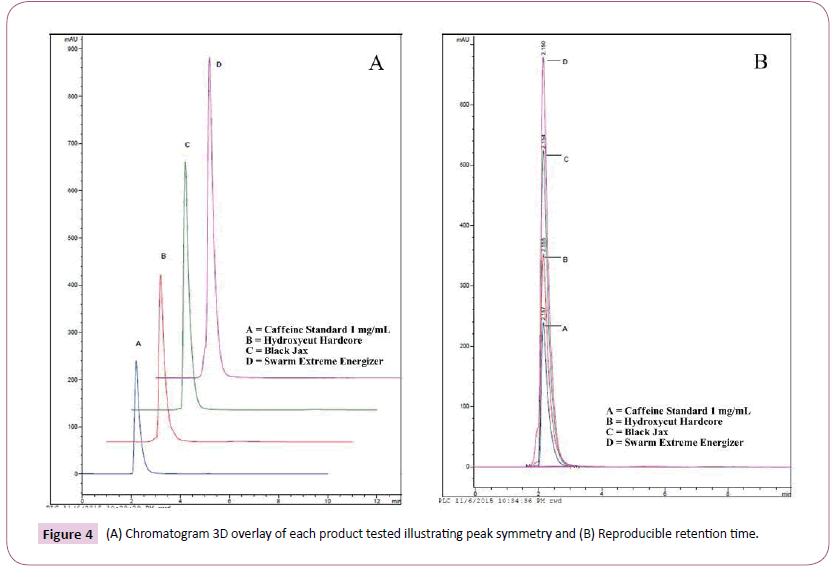
As previously described, the entire contents of each capsule were emptied into a graduate cylinder followed by the addition of hot water to a final volume of 100 mL for maximum caffeine solubility. Using the stated isocratic method of water: acetonitrile (40:60) a reproducible caffeine peak was obtained with a retention time of 2.15 min. This retention time was consistent for all products investigated. Figure 3 depicts peaks and retention times for A) caffeine standard, B) Hydroxy-Cut, C) Black Jax, and D) Swarm. Figure 4A affords a 3D overlay of the caffeine standard and each of the three supplements investigated while Figure 4B represents the overlay of caffeine peaks from standard and each supplement. Retention time for caffeine from all supplements was routinely 2.15 minutes. Area under the curve data was obtained and used for calculating caffeine concentrations for each product. Table 1 compares caffeine content of the three products based on product labelling and measured concentrations. Of the three supplements investigated, all but Hydroxy-Cut were roughly within ± 10% the stated value on the product label. Hydroxy-Cut, with a proprietary source of caffeine, was consistently more than 20% above the stated value on the label.
Discussion
Caffeine consumption through ingestion of coffee, tea, soda, energy drinks, and oral supplements in United States is significant and represents a growing health concern. Numerous reports of adverse events owing to caffeine consumption can be found in the literature and appear to be on the rise [10,11,14,19-26]. While caffeine-related adverse events are often reversible, fatalities have been attributed to unintentional caffeine consumption [11,19,21]. One reason for the apparent increase in caffeinerelated adverse events may well be the general availability of caffeinated beverages. Additionally, caffeine-containing oral supplements marketed as energy boosters or weight loss supplements are widely available in numerous retail settings. It is very likely that consumers are unaware of how much caffeine they are consuming on a daily basis [27]. Caffeine content in OTC oral products is typically not regulated nor are quality assurance standards required as these products make no specific therapeutic claim. Despite disclosure of caffeine content on the product label, consumers should be cautious of the actual versus stated amount indicated on the label. The present study investigated three products readily available at gas stations, large retailers, and nutrition centers for caffeine content. A reverse-phase HPLC method attempted to validate the stated caffeine content for each product. The products investigated, Swarm, Black Jax, and Hydroxy-Cut, are all readily available over the counter. The stated caffeine content in each capsule was 300 mg for Swarm, 250 mg for Black Jax, and 135 mg for Hydroxy-Cut. The AUC data obtained from sample analysis suggests that the actual caffeine content for Swarm and Black Jax is close to the stated value on the product with Black Jax being close to 2% below the stated value and Swarm being 11% above the stated value. However, Hydroxy-Cut has closer to 160 mg of caffeine per capsule which is greater than 20% more caffeine than stated on package labelling. Table 1 illustrates the average concentration values obtained using AUC data for 6 individual runs for each supplement tested as well as the percentage difference between stated and actual caffeine content. Keeping in mind that pharmaceutical products must be within ± 10% of the stated potency, Black Jax and Swarm contained close to the labelled caffeine content despite not being regulated. The additional caffeine detected in Hydroxy- Cut is not surprising. It is possible that the manufacturer of Hydroxy-Cut produces capsules with additional caffeine in order to compensate for degradation. However, caffeine is a rather robust small molecule and is not noted to undergo decomposition unless subjected to harsh alkaline conditions. When exposed to 1N sodium hydroxide for a brief period of time we were able to detect degradation of caffeine (data not shown). Proper storage of product should prevent degradation thus obviating the need to add additional caffeine to any product. However, the label for Hydroxy-Cut lists a “proprietary blend” of ingredients, some of which may contribute to the additional caffeine detected. Natural sources of caffeine including tea leaves, yerba mate and/or guarana may contain varying amounts of caffeine. When these botanicals are combined with known amounts of caffeine to produce supplements, the detected amount of caffeine has been reported to be significantly higher than similar products without botanical [28]. It is reasonable to suggest that the blend of botanicals used in Hydroxy-Cut is contributing to the higher than expected caffeine content in this product.
| Products | Theoretical concentration (mg/mL after dilution) | Detected concentration (mg/mL) | Percent difference |
|---|---|---|---|
| Caffeine standard | 1 | 0.98 | -2.2 |
| Hydroxy cut | 1.35 | 1.64 | 23.2 |
| Black Jax | 2.5 | 2.49 | -1.8 |
| Swarm | 3 | 3.31 | 11 |
Table 1: The average concentration values obtained as well as the percentage difference between stated and actual caffeine content.
Caffeine is ubiquitous in society and consumed in numerous forms. Beverages including Mountain Dew®, Pepsi®, and Coke® have stated caffeine concentrations of up to 55 mg, 39 mg, and 35 mg per serving, respectively. The caffeine content in energy drinks such as 5-hour Energy® and Red Bull® are reported to be 207 mg and 80 mg of caffeine per serving, respectively. This amount of caffeine is consistent with typical consumption from coffee and otherwise not concerning or expected to result in any severe adverse effects. What is concerning however, is the rate at which an individual can ingest significant amounts caffeine. It is certainly possible to ingest several cans of soda or several 5-Hour Energy drinks in a short period of time. Ingesting 4 capsules of Swarm, which would amount to a total of 1200 mg caffeine, could also easily be accomplished in a rapid fashion. More concerning is that these oral products may be ingested using a caffeinated beverage. While the Food and Drug Administration has proposed measures to reduce the risk of adverse effects associated with energy drink consumption, additional measures are required to help educate society on the dangers of excessive caffeine intake and the products that pose the greatest risk [29]. While this study demonstrated that some caffeinated supplements have good agreement between the stated content and actual caffeine content, products may exist which have excessive caffeine or other toxic substances putting the consumer at risk for adverse effects. Accurate labels and information on the safe use of caffeine is needed for the public. In addition, strategies need to be developed to prevent unnecessary adverse effects from excessive caffeine ingestion [27].
Conclusion
In conclusion, this study involved the development of an HPLC method to identify and quantitate caffeine in several readily accessible supplements. Calculated caffeine values derived from AUC data were compared to stated values on supplement labels. Of the three supplements investigated, the caffeine content detected in Hydroxy-Cut was consistently 20% above the stated value. It is quite possible that the use of natural sources of caffeine in supplements contributes to the variability in actual caffeine content as noted in Hydroxy-Cut. Given that supplements are not subject to rigorous quality standards, it is critical for consumers to be aware of the actual amount of caffeine they may be ingesting. Likewise, data showing that a product is devoid of a stated ingredient would be just as critical. While this study only looked at three supplements available from online vendors or gas stations, numerous supplements remain widely available to the public which creates concern and opportunity at the same time. More analysis of supplements is required in order to keep the best interest of the public.
Acknowledgements
The authors would like to thank Dr. Paluri Sai Shantanu Rao for careful review of this manuscript. Support for this project was provided by the College of Pharmacy at The University of Findlay.
Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83-133.
- Fenton RA, Poulsen SB, De La Mora Chavez S, Soleimani M, Busslinger M, et al. (2015) Caffeine-induced diuresis and natriuresis is independent of renal tubular NHE3. Am J Physiol Renal Physiol 308: 1409-1420.
- Daniels JW, Mole PA, Shaffrath JD, Stebbins CL (1998) Effects of caffeine on blood pressure, heart rate, and forearm blood flow during dynamic leg exercise. J Appl Physiol 85: 154-159.
- Kaplan GB, Greenblatt DJ, Ehrenberg BL, Goddard JE, Cotreau MM, et al. (1997) Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol 37: 693-703.
- Doyle J, Davidson D, Katz S, Varela M, Demeglio D, et al. (2016) Apnea of prematurity and caffeine pharmacokinetics: potential impact on hospital discharge. J Perinatol 36: 141-144.
- Julien CA, Joseph V, Bairam A (2010) Caffeine reduces apnea frequency and enhances ventilatory long-term facilitation in rat pups raised in chronic intermittent hypoxia. Pediatr Res 68:105-111.
- Ribeiro JA, Sebastiao AM (2010) Caffeine and adenosine. J Alzheimers Dis 20 Suppl 1: S3-15.
- Zhang YG, Shen L, Xu L, Huang YG (2014) Regulatory effect of caffeine on the acute and the chronic pain and its possible mechanisms. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Academiae Medicinae Sinicae 36: 697-700.
- Becker WJ (2015) Acute migraine treatment in adults. Headache 55: 778-793.
- Ciszowski K, Biedron W, Gomolka E (2014) Acute caffeine poisoning resulting in atrial fibrillation after guarana extract overdose. Przegl Lek 71: 495-498.
- Jabbar SB, Hanly MG (2013) Fatal caffeine overdose: A case report and review of literature. Am J Forensic Med Pathol 34: 321-324.
- Klatsky AL, Hasan AS, Armstrong MA, Udaltsova N, Morton C (2011) Coffee, caffeine, and risk of hospitalization for arrhythmias. Perm J 15: 19-25.
- Lee SM, Choi NK, Lee BC, Cho KH, Yoon BW, et al. (2013) Caffeine-containing medicines increase the risk of hemorrhagic stroke. Stroke 44: 2139-2143.
- Thyagarajan B, Alagusundaramoorthy SS, Agrawal A (2015) Atrial fibrillation due to over the counter stimulant drugs in a young adult. J Clin Diagn Res 9: 05-07.
- Wolk BJ, Ganetsky M, Babu KM (2012) Toxicity of energy drinks. Curr Opin Pediatr 24: 243-251.
- Rosenfeld LS, Mihalov JJ, Carlson SJ, Mattia A (2014) Regulatory status of caffeine in the United States. Nutr Rev 72 :23-33.
- Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R (2012) Association of coffee drinking with total and cause-specific mortality. N Eng J Med 366: 1891-1904.
- The Merck Index (2013) An encyclopedia of chemicals, drugs and biologicals (15th edn). M.J. O’Neil, (ed). The Royal Society of Chemistry, Cambridge, UK.
- Banerjee P, Ali Z, Levine B, Fowler DR (2014) Fatal caffeine intoxication: A series of eight cases from 1999 to 2009. J Forensic Sci 59: 865-868.
- Beauchamp GA, Johnson AR, Crouch BI, Valento M, Horowitz BZ (2016) A retrospective study of clinical effects of powdered caffeine exposures reported to three US poison control centres. J Med Toxicol 12: 295-300.
- Bonsignore A, Sblano S, Pozzi F, Ventura F, Dell'Erba A (2014) A case of suicide by ingestion of caffeine. Forensic Sci Med Pathol 10: 448-451.
- Davison G, Shoben A, Pasch KE, Klein EG (2016) Energy drink use among Ohio Appalachian smokers. J Community Health 41: 897-902.
- Gunja N, Brown JA (2012) Energy drinks: Health risks and toxicity. Med J Aust 196: 46-49.
- Lystrup RM, Leggit JC (2015) Caffeine toxicity due to supplement use in caffeine-Naive individual: A cautionary tale. Mil Med 180: e936-e940.
- Pendleton M, Brown S, Thomas CM, Odle B (2013) Potential toxicity of caffeine when used as a dietary supplement for weight loss. J Diet Suppl 10: 1-5.
- Pennay AE, Lubman DI (2012) Energy drinks: Health risks and toxicity. Med J Aust 196: 442.
- Kole J, Barnhill A (2013) Caffeine content labeling: A missed opportunity for promoting personal and public health. J Caffeine Res 3: 108-113.
- Andrews KW, Schweitzer A, Zhao C, Holden JM, Roseland JM, et al. (2007) The caffeine contents of dietary supplements commonly purchased in the US: analysis of 53 products with caffeine-containing ingredients. Anal Bioanal Chem 389: 231-239.
- Thorlton J, Colby DA, Devine P (2014) Proposed actions for the US Food and Drug Administration to implement to minimize adverse effects associated with energy drink consumption. Am J Public Health 104: 1175-1180.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences