Chemo-preventive Properties of Essential Oils Obtained from Boswellia carterii and B. sacra in Combination with Insoluble β-Glucan
Olivier Fortin , Blanca Aguilar-Uscanga , Khanh Dang Vu , Stephane Salmieri , Jing-Cheng Zhao and Monique Lacroix
Olivier Fortin1, Blanca Aguilar-Uscanga2, Khanh Dang Vu1, Stephane Salmieri1, Jing-Cheng Zhao1 and Monique Lacroix1*
1INRS-Institute Armand-Frappier, Research Laboratories in Sciences Applied to Food, Canadian Irradiation Centre, The Institute of Nutraceutical and Functional Foods, Boulevard Des Prairies, Laval, QC, Canada
2Laboratorio de Microbiología Industrial, Centro Universitario de Ciencias Exactas e Ingenierías, Universidad de Guadalajara (UdG), Marcelino García Barragan Guadalajara, Jalisco, Mexico
- *Corresponding Author:
- Dr. Monique Lacroix
Professor at INRS-Institute Armand- Frappier
Research Laboratories in Sciences Applied to Food
Canadian Irradiation Centre, The Institute of Nutraceutical and Functional Foods (INAF)
531, Boulevard des Prairies, Laval, Québec, Canada H7V 1B7.
Tel: +450 687 5010 #4489
Fax: +450 686 5501
E-mail: monique.lacroix@iaf.inrs.ca
Received Date: September 25, 2017; Accepted Date: October 05, 2017; Published Date: October 20, 2017
Citation: Fortin O, Aguilar-Uscanga B, Vu KD, Salmieri S, Zhao JC, et al. (2017) Chemo-preventive Properties of Essential Oils Obtained fromBoswellia carterii and B. sacra in Combination with Insoluble β-Glucan. J Nutraceuticals Food Sci. Vol. 2 No.3:13
Abstract
Background: B. carterii (Frankincense) and B. sacra (Sacred) essential oils (EOs) have been used for many centuries in several medicinal applications. More recently, those EOs were investigated for their anticancer properties and used in combination with other natural compounds resulting in enhanced biological activities. To that extend, yeast-derived β-glucan have shown remarkable anticancer and chemo-preventive potential in the past decades whether tested alone or in combination. In this context, the in vitro chemo-preventive, antiradical and antiproliferative effects of B. carterii (Frankincense) and B. sacra (Sacred) EOs used alone and in combination with Insoluble β-glucan from Saccharomyces boulardii on colorectal cancer (CRC) were investigated.
Methods and findings: Essential oils and insoluble β-glucan from Saccharomyces boulardii cell wall were assayed for their capacity to increase the specific activity of NAD(P)H: quinone reductase (QR), scavenge radicals and inhibit growth of human CRC cells (CHO-K1 and HT-29 cells). Results demonstrated that B. carterii (Frankincense) and B. sacra (Sacred) scavenged superoxide anions and similarly inhibited growth of two human CRC cell lines. This study also reported the increase of QR activity as a novel mechanism of action of these EOs in cancer prevention and demonstrated that insoluble β-glucan from S. boulardii cell wall enhanced the capacity of B. carterii (Frankincense) EO to increase QR specific activity as oppose to Sacred oil. Finally, Sacred oil efficiently scavenged superoxide anions and expressed cancerous cell-specific cytotoxicity when opposed to B. carterii (Frankincense) EO.
Conclusion: Results obtained in this study represents the first evidence thatBoswellia EOs can enhance QR activity when used alone or in combination with insoluble β-glucan from S. boulardii cell wall, hence suggesting a novel combination to investigate in future investigations. Complete characterization ofBoswellia EOs and further biological analyses will be required to identify component(s) that potentially responsible for such chemoprevention activities.
Keywords
Boswellia essential oils; Chemoprevention; Anti-radical; Antiproliferative; NAD(P)H: Quinone reductase; β-glucan; Apoptosis
Abbreviations
EOs: Essential Oils; 5-FU: 5-Fluorouracil; CRC: Colorectal Cancer; MTT:3-(4,5dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide; NADP: Nicotinamide Adenine Dinucleotide Phosphate; QR: NAD(P)H: Quinone Reductase; X/XO: Xanthine/Xanthine Oxidase; DPPH: Α,Α-Diphenylβ-Picrylhydrazyl; PI: Propidium Iodide; CI: Combination Index; IC50: Concentration that Inhibits 50% of the Cellular Growth; SC50: Concentration that Scavenges 50% of Formed Radicals.
Introduction
The colorectal cancer (CRC) is the second most deadly cancer in males and the third for females [1] and is the third most prevalent cancer in Canada [2]. The increase of CRC in Canada and USA has generated an increased interest in the consumption of natural products to prevent the development of this disease. Thus, prevention seems to be the most efficient approach since treatments for CRC can be expensive and invasive for patients. Chemoprevention consists of using natural or synthetic materials to prevent the progression of cancer [3]. Many essential oils (EOs) and their constituents have been reported to be chemopreventive agents due to their abilities to affect phase I and II enzymes, prevent lipid peroxidation, suppress cyclooxygenase-2 activity and exhibit anticancer properties such as in vivo antitumoral activities, apoptosis and cancerous cell specific cytotoxicity [4,5]. More specifically, EOs fromBoswellia spp. are well known for their tumor cell specific cytotoxicity and their capacity to induce apoptosis in cancerous cells [6,7]. In this aspect, the use ofBoswellia spp. EOs as chemo-preventive agents toward CRC appears to be relevant. Moreover, EOs exhibiting enhanced chemo-preventive properties obtained by combination with known chemo-preventive agents is also a relevant approach to reduce CRC development.
EOs obtained fromBoswellia trees have been used for many centuries in religious rituals and medicinal applications such as inflammation, immune support, skin health and more recently cancer treatment. As found with others EOs, the biological properties of B. carterii (Frankincense) and B. sacra (Sacred) EOs vary according to many factors such as plant species, plant organs, extraction methods, soil composition, vegetative cycle stage and climate of harvesting [8]. In this context, many scientists, botanists and governments tend to consider B. carterii and B. sacra as the same species whereas several studies tend to prove the opposite using chemical characterization [9]. Despite these evidences, very few studies have investigated differences between B. carterii (Frankincense) and B. sacra (Sacred) EOs regarding their biological activities toward colorectal cancer (CRC), which may serve to deepen knowledge on chemopreventive properties of these EOs.
Yeast cell walls mainly consist of mannoprotein, chitin and (1→3)-β-D-glucan with (1→6)-β-D-glucan ramifications [10]. Yeast insoluble β-glucan are known for their strong immunomodulatory properties [11] and can be easily extracted from spent yeast [12]. Furthermore, several investigations revealed the capacity of yeast-derived β-glucan to prevent and treat different types of cancer both in humans and rats [11,13]. Those properties depend on physicochemical nature and integrity of the β-glucan structure, which vary according to growth conditions, extraction methods, and yeast species [14-16]. More recently, the chemopreventive potential in vitro and in vivo of insoluble β-glucan from S. boulardii cell wall was demonstrated. Notably, this specific extract appeared to be an inducer of NAD(P)H: quinone reductase (QR), a phase II detoxification enzyme (EC 1.6.99.2) in vitro and in vivo and could significantly reduce aberrant crypt count in 1,2-dimethylhydrazine-treated rats [17,18].
To substantiate differences between B. carterii (Frankincense) and B. sacra (Sacred) EOs, this study investigated the differences and the mechanism of action of B. carterii (Frankincense) and B. sacra (Sacred) EOs regarding chemo-preventive, antiradical and antiproliferative properties toward CRC. In this context, EOs were evaluated for their capacity to induce QR activity, which has never been evaluated before, and to scavenge superoxide anions (O2-) and DPPH radicals, which are known to be involved in CRC carcinogenesis. Moreover, EOs were tested for their antiproliferative activities against cancerous and non-cancerous cells to reveal a cancerous cell specific cytotoxicity then an apoptosis assay was conducted to determine if this mechanism was involved. Finally, efforts were successfully invested to enhance biological activities of EOs through addition of insoluble β-glucan to B. carterii (Frankincense) and B. sacra (Sacred) EOs.
Methods
Chemicals
Chemicals and media were obtained as follows: essential amino acids, sodium pyruvate, fetal bovine serum (FBS), minimum essential medium-Earle’s balanced salt solution (MEM-EBSS), Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (MEMF/12), Ham’s F-12 medium, Dulbecco's Modified Eagle Medium low glucose, Hank’s balanced salt solutions (HBSS), trypsin, Pierce®BCA Protein assay, glycine, 25 cm2 flask, 96-well and 6-well microplates were purchased from Fisher Scientific (Ottawa, ON, Canada). Activated carbon, digitonin, bovine serum albumin (BSA), glucose-6phosphate, thiazolyl blue tetrazolium bromide (MTT), menadione, glucose-6-phosphate dehydrogenase, nicotinamide adenine dinucleotide phosphate (NADP) and flavin adenine dinucleotide (FAD), Tween80, sodium azide, 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium- 5-carboxanilide inner salt (XTT), xanthine, sodium carbonate buffer (pH 10∙2), xanthine oxidase, superoxide dismutase, Nmethylpyrrolidone (NMP), α,α-diphenyl-β-picrylhydrazyl (DPPH) and 5-fluorouracil (5-FU) were purchased from Sigma- Aldrich (Oakville, ON, Canada). Lecithin was purchased from ADM (Calgary, AB, Canada). B. carterii (Frankincense) and B. sacra (Sacred) EOs were graciously provided by Young Living Essential Oils (Lehi, UT, USA). Annexin V-FITC/PI Dead Cell Apoptosis kit was purchased from Invitrogen (Burlington, ON, Canada). Polymethyl methacrylate (PMMA) was obtain from Agilent technologies (Mississauga, ON, Canada).
EOs preparation
Boswellia carterii and B. sacra were harvested in Kenya and Oman respectively to obtain Frankincense and Sacred EOs using the steam distillation method and were kindly provided by Young Living Essential Oils (Lehi, UT, USA). B. carterii (Frankincense) and B. sacra (Sacred) EOs were prepared under oil-in water emulsion containing 1% (v/v) Tween-80 and 1% (w/v) lecithin as emulsifying agents. More specifically, Tween-80 and lecithin were solubilized into water using magnetic stirrer resulting in a solution termed emulsifying solution. Then, EOs were added slowly to the stirred solution until a final concentration of 68800 ppm was reached. The O/W emulsion was covered from light and roughly stirred until complete homogenization (approximatively 45 min). Then, the resulting emulsion was filtered through a 0.2 μm filter. In order to assay various concentrations of EOs, this emulsion was diluted with sterile emulsifying solution until desired concentration under sterile condition. For combined treatments containing EOs and insoluble β-glucan, an emulsion was prepared as mentioned above but filtration was replaced by the addition of sodium azide (20 ppm) to prevent microbial contamination. The addition of pre-weighted insoluble β-glucan (40000 ppm) from S. boulardii cell wall and SA under sterile condition was performed following complete homogenization of EO in emulsifying solution then stirred for another 5 min. For antiradical assays, EOs were serial diluted in anhydrous ethanol to a final concentration of 34400 ppm.
Extraction of insoluble β-glucan from S. boulardii cell wall
Extraction of insoluble β-glucan was performed as described by Fortin, Aguilar-Uscanga, Vu, Salmieri and Lacroix [18]. Briefly, S. boulardii cells were grown in yeast peptone media containing 1% (w/v) dextrose and collected in early stationary phase. The cell suspension was centrifuged at 9000 × g for 10 min at 4°C and the resulting biomass was washed twice with sterile phosphate buffer 50 mM, pH 7∙2. Then, the wet biomass was suspended in sterile water (15% w/v) and autolyzed for 24 h at 50°C under agitation at 200 rpm. The autolyzed biomass was then centrifuged at 9000 × g for 10 min at 4°C and 500 ml of 1 mol/l NaOH was mixed with 100 g of wet autolyzed cells for 1 h at 90°C without agitation. Finally, the resulting suspension was centrifuged as described above and the precipitate was washed twice with distilled water and then freeze-dried.
Cell lines and cells maintenance
Hepa 1c1c7 ATCC CRL-2026, HT-29 ATCC HTB-38, CHO-K1 and CaCO2 cell lines were purchased from American type culture collection (ATCC) (Manassas, VA, USA). All cell lines were cultivated in 25 cm2 cellular flasks (Corning, NY, USA) at 37°C in a humidified incubator with an atmosphere of 5% CO2 and 95% air. Hepa 1c1c7 and HT-29 cells were grown in complete MEMEBSS and complete MEMF/12 media, respectively (0.1% essential amino acids, 0.1% sodium pyruvate, 10% FBS). CaCO2 cells were grown in Dulbecco's Modified Eagle Medium low glucose (0.1% essential amino acids, 0.1% sodium pyruvate, 20% FBS) and CHO-K1 cells were grown in Ham’s F-12 media (20% FBS). At a confluence of 80% to 90%, cells were treated with 1X trypsin- EDTA for 12 min at 37°C in presence of 5% CO2. Finally, trypsin was inactivated with 2 ml of respective media and 1 ml of the resulting suspension was used to inoculate 5 ml of fresh media.
Anti-radical assays (O2- and DPPH radicals scavenging activity)
The capacity of B. carterii (Frankincense) and B. sacra (Sacred) EOs to scavenge O2- anions was measured using the xanthine/ xanthine oxidase (X/XO) system (XTT color assay) based on Gerhäuser, Klimo, Heiss, Neumann, Gamal-Eldeen, Knauft, Liu, Sitthimonchai and Frank [19] with modification. A 20 μl sample previously diluted in ethanol was loaded in a 96-well microplate and completed to 200 μl with reactional mix (1 mmol/l XTT, 1 mmol/l EDTA, 1 mmol/l xanthine, 50 mmol/l sodium carbonate buffer (pH 10∙2) and 3 mU/ml xanthine oxidase). The optical density (OD) was read at 490 nm after 20 min. Negative and positive controls consisted of ethanol and 30 U/ml of superoxide dismutase respectively. Scavenging activity was calculated as follows:
% Scavenging Activity = [(sample OD - Negative control OD)/ (Positive control OD - Negative control OD)] × 100 (1)
The capacity of B. carterii (Frankincense) and B. sacra (Sacred) EOs to scavenge DPPH was based on the method of Blois and Kedare and Singh with some modifications [20,21]. Briefly, 1 ml of 40 μM DPPH previously dissolved in anhydrous ethanol was added to 250 μl of serial diluted EOs (also diluted in anhydrous ethanol). The solution was mixed and kept at room temperature for 1 hour protected from lights then, optical density was read at 517 nm. The blank consisted of 1.25 ml anhydrous ethanol whereas control consisted of 250 μl of anhydrous ethanol and 1 ml of DPPH solution. The inhibition percentage (IP) of free radicals was measured by the equation proposed by Megdiche- Ksouri, Trabelsi, Mkadmini, Bourgou, Noumi, Snoussi, Barbria, Tebourbi and Ksouri [22]:
IP (%) = ([Control OD – Sample OD]/Control OD) × 100 (2)
For both assays, concentrations that exhibited a scavenging activity of 50% (SC50 values) were determined.
NAD(P)H: QR assay
QR assay was based on methods from Prochaska and Santamaria and Talalay with some modifications [23,24]. Hepa 1c1c7 cells were seeded at a density of 2 ×103 cells/well in a 96-well plate using complete MEM-EBSS media and were incubated at 37°C in a humidified incubator with 5% CO2. Afterward, the media was removed using a multichannel micropipette and serial diluted samples were added, then the microplate was incubated for 48 h as mentioned above. Cells were washed with 200 μl HBSS solution and 50 μl of 1.6% (w/v) digitonin were added to each well followed by a 20-min incubation. Then, 20 μl of samples were removed using a multichannel micropipette and used for total protein quantification whereas 200 μl of complete reaction mixture (0.25 mol/l Tris-HCl pH 7, 4.67% (w/v) BSA, 0.01% Tween-20, 5 mol/l FAD, 1 mmol/l glucose-6-phosphate, 30 mol/l NADP, 34.8 μmol/l MTT, 50 μmol/l menadione and 2 mU/μl glucose-6-phosphate dehydrogenase) were added to each well then incubated at room temperature for 5 min. The microplate was read at 595 nm. A protein assay was conducted using Pierce®BCA reagents using the manufacturer’s instruction. Controls consisted of emulsifying solution whereas media was used as blank. Specific activity of QR was defined as nmol of blue formazan formed per mg protein per minute. Fold induction of QR was calculated as follows:
Fold induction = Specific Activity of Treated Group/Specific Activity of Negative Control Group (3)
Molecular weight determination by gel permeation chromatography (GPC)
Molecular weights (Mw) of insoluble β-glucan treated with B. sacra (Sacred) EO was analyzed by gel permeation chromatography (GPC) (Agilent Technologies 1260 infinity series, Waldbronn, BW, Germany), equipped with a quaternary pump (Model G1311B), a manual injector with a sample loop of 20 μl and a refractive index detector (Model G1362A). Two identical PL gel 5 μm Mixed-D 300 × 7.5 mm columns were used in series and mobile phase consisted of 100% N-methylpyrrolidone (NMP) containing 5% (w/v) LiCl at a flow rate of 0.5 ml/min. Both columns and detector were set at 60°C. Insoluble β-glucan (5 mg) and EOs were suspended in 5 ml of emulsion as described in section 2.2. in a proportion of 5:1 for 48 h. Then, 2 ml of 100% NMP was added to obtain a relative concentration in insoluble β-glucan of 2.5 mg/ml and the suspension was stirred for 48 h at 60°C, filtered through a nylon 0.2-μm filter and injected in the column. Polymethyl methacrylate (PMMA) was used as a standard and was prepared as indicated by the manufacturer. The equation obtained by plotting Mw with retention times of standards was used to calculate Mw of insoluble β-glucan. All extracts were injected in triplicate (n=3).
Anti-proliferative assay
Antiproliferative properties were determined by the ability of the metabolic active cells to cleave the tetrazolium salt to purple formazan crystals based on Vistica, Skehan, Scudiero, Monks, Pittman and Boyd [25]. Different cell lines were seeded at 2 × 104 cells/well of media in a 96-well plate (200 μl/well) and were incubated for 24 h at 37°C in 5% CO2. Then, spent media were removed using a multichannel micropipette. A quantity of 100 μl of fresh media containing 10 μl of sample previously serial diluted (ranging from 3000 to 5.85 ppm regarding both EOs and insoluble β-glucan) was immediately added in well and the microplate was then incubated for 48 h as mentioned above. Afterward, samples were removed using a multichannel micropipette and replaced with 225 μl of fresh media containing 25 μl 0.5% (w/v) MTT followed by incubation for 4 h at 37°C in 5% CO2. Finally, the media was carefully removed using a multichannel micropipette and replaced by 225 μl of DMSO containing 25 μl of Sorensen buffer containing 0.1 mol/l glycine and 0.1 mol/l NaCl at a pH of 10.5. The microplate was then read at 562 nm. The negative control and blank consisted of emulsifying solution and media, respectively. Growth inhibition was calculated as follows:
% Growth inhibition = 100 – (([Sample OD]/Negative control OD) × 100) (4)
Equations obtained by plotting the linear portion of growth inhibition versus increasing concentrations of samples were used to calculate concentrations that inhibit 50% of cellular growth (IC50 values). For combined treatments, concentrations corresponding to IC50 values when tested separately were serial diluted and assayed.
Assessing interaction between EOs and insoluble β-glucan regarding antiproliferative and NAD(P) H: QR assays
The assessment of interactions in combined treatments regarding QR and antiproliferative assays differed due to the nature of measured effects. The determination of combined effects concerning antiproliferative assay was based on combination index (CI) as used by Hossain, Follett, Dang Vu, Harich, Salmieri and Lacroix [26] with different upper and lower bounds suggested by Berenbaum [27] following the equation:
CI = [Dx/IC50x] + [Dy/IC50y] (5)
where Dx and Dy represent concentrations of components used in combination that reached IC50 values whereas IC50x and IC50y represents concentrations of components x and y that reached IC50 values when tested separately. Based on CI values, different combined effects can be classified: CI value<1 was interpreted as a synergistic effect, a CI value equal to 1 was interpreted as an additive effect and a CI>1 was interpreted as an antagonistic effect. Concerning the QR assay, concentrations that exhibited an induction of 1.5 when tested separately were used for combined treatment assays. Determination of the combined effect was based on fold induction and assessed as follows: Fold induction of 1.5 to 3.0 was interpreted as an additive effect, fold induction ≈1.5 as no interactive effect and fold induction <1.5 as an antagonistic effect. Fold induction calculated in combined treatments was obtained as described in equation 3.
Apoptosis assay
HT-29 cells were seeded in a 6-well plate at 3 × 105 cells/well (3 ml/well) and incubated as described in section 2.8. for 24 h. Then, cells were incubated for 48 h at 37°C in 5% CO2 in the presence of 450, 900 and 1800 ppm of B. carterii (Frankincense) and B. sacra (Sacred) EOs in a final volume of 3 ml to surround IC50 values obtained for this cell line. Cells present in the supernatant were harvested by centrifugation at 500 × g for 10 min at 4°C. Adhered cells were treated with 1 ml of 1x trypsin-EDTA for 12 min at 37°C. Then, 2 ml of complete MEM/F12 medium was added and cells were harvested by centrifugation as described previously. The cell-containing pellets (from the supernatant and the adhered cells) were washed twice with PBS containing 0.25% EDTA to avoid clumping and the apoptosis evaluation was performed by using Annexin V-FITC and PI double staining assays. Harvested cells were diluted in 1X binding buffer at 106 cells/ ml and Annexin V-FITC/PI staining was performed according to the manufacturer’s instructions with a total of 10,000 events by flow cytometry (Coulter Epics XL-MCL, Beckman Coulter Canada, Inc., Mississauga, ON, Canada). The 5-fluouracil (5-FU) was used as positive control and emulsifying solution was used as negative control.
Statistical analysis
All measurements were done in triplicate (n=3) and results are presented as average ± standard deviation. QR fold induction, IC50 values and percentage of apoptotic and necrotic cells were analyzed by one-way analysis of variance (ANOVA) using PASW statistics 18 software (IBM Corporation, Somers, NY, USA) and differences among treatments were analyzed with a post hoc Duncan’s multiple-range test.
Significance was considered at P ≤ 0.05.
Results
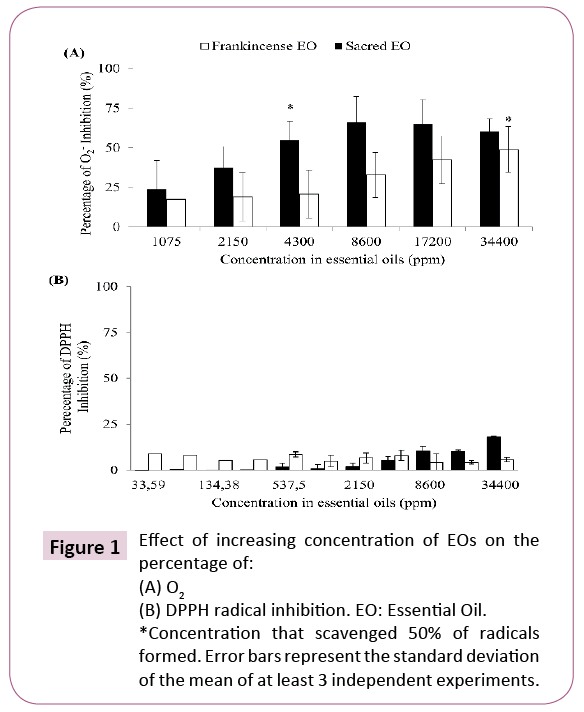
Evaluation of the anti-radical properties of EOs
Antiradical properties of EOs fromBoswellia spp. were investigated via their capacity to scavenge O2- and DPPH radicals and the results are presented in Figure 1 B. carterii (Frankincense) and B. sacra (Sacred) EOs demonstrated a dosedependent response in antiradical activities and were found to scavenge 50% of O2- anion at 4300 and 34400 ppm respectively in addition to demonstrate a dose dependent response (Figure 1A). These results are suggesting that B. sacra (Sacred) EO possess an enhanced capacity to scavenge O2- anion as compared to B. carterii (Frankincense) EO. In contrast, both EOs were not able to scavenge 50% of DPPH radical despite their high concentration ranging from 33.59 to 34400 ppm. However, a dose-dependent response was also observed suggesting a weak capacity of B. sacra (Sacred) EO (18% at 34400 ppm) to scavenge DPPH radical (Figure 1B).
Effect of EOs in combination with insoluble β-glucan on the induction of NAD(P)H: QR and molecular weight
To determine the chemo-preventive potential of B. carterii (Frankincense) and B. sacra (Sacred) EOs, their effects on QR activity was evaluated and results are shown in Table 1. Cells treated with emulsifying solution (control) showed a 0.8-fold induction which corresponded to a basal expression of QR in Hepa 1c1c7 cells. Moreover, B. carterii (Frankincense) and B. sacra (Sacred) EOs reached maximum fold inductions of 1.5 and 1.4 at a concentration of 27 and 54 ppm respectively. In addition, both EOs tested separately demonstrated a maximum fold induction significantly higher than control (0.8-fold induction) (P ≤ 0.05). Those results demonstrated that B. carterii (Frankincense) EO (27 ppm) is twice as efficient as B. sacra (Sacred) EO (54 ppm) to induce QR. In order to increase the chemo-preventive potential of B. carterii (Frankincense) and B. sacra (Sacred) EOs, they were combined with insoluble β-glucan of S. boulardii which has demonstrated an excellent chemo-preventive potential against colorectal cancer evaluated in vitro and in vivo [17,18]. Concentrations in EOs and insoluble βglucan that exhibited similar fold inductions were mixed to determine the combined effect of EO + insoluble β-glucan on QR activity and these results are also presented in Table 1. The combination of B. sacra (Sacred) EO+ B. carterii (Frankincense) EO showed a similar QR activity (1.4- fold induction) as compared to each EO tested separately, hence suggesting there was no interactive effect between two EOs. In contrast, the combination of B. sacra (Sacred) EO + insoluble β-glucan revealed a QR activity (0.4-fold induction) which was significantly (P ≤ 0.05) lower than of B. sacra (Sacred) EO (1.4- fold induction) and insoluble β-glucan (1.5-fold induction at 250 ppm) alone, hence suggesting an antagonistic effect. The combination of B. carterii (Frankincense) EO + insoluble β-glucan revealed a QR activity (2.0-fold induction) which was significantly (P ≤ 0.05) higher than that of B. carterii (Frankincense) EO (1.5- fold induction) or insoluble β-glucan (1.5-fold induction) alone, hence suggesting an additive effect. These results demonstrated that insoluble β-glucan improves the capacity of B. carterii (Frankincense) EO in inducing QR activity. The antagonistic effect of B. sacra (Sacred) EO when combined with insoluble β-glucan on QR activity led to a hypothesis that B. sacra (Sacred) EO might degrade insoluble β-glucan. The hypothesis was verified by comparing the molecular weight (Mw) of insoluble β-glucan before and after addition of B. sacra (Sacred) EO. Results demonstrated that insoluble β-glucan possessed a Mw (1921 ± 13 kDa) similar as if it was combined with B. sacra (Sacred) EO (1904 ± 297 kDa), hence suggesting that EOs did not affect the insoluble β-glucan.
| Samples | IC50 (ppm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HT-29 | Caco2 | CHO-K1 | ||||||||||
| Insoluble glucan | Total EOs | CI | CE | Insoluble glucan | Total EOs | CI | CE | Insoluble ‡ glucan |
‡ Total EOs |
CI | CE | |
| Sacred EO | NA | 1348 ± 107B | NA | NA | 1138 ± 57B | NA | NA | NR | NA | NA | ||
| Frankincense EO | NA | 1447 ± 86B | NA | NA | 1424 ± 206A,B | NA | NA | 1689 ± 22B | NA | NA | ||
| Insoluble β-glucan | 108 ± 33a | NA | NA | 634 ± 242a,b,c | NA | NA | NR | NA | NA | NA | ||
| Frankincense + Sacred EOs | NA | 1465 ± 276A,B | 1.05 | AD | NA | 1032 ± 8A,B | 0.82 | AD | NA | 704 ± 108A | CI<1.0 | S |
| Insoluble β-glucan + Sacred EO | 93 ± 6a | 1279 ± 87B | 2.61 | A | 307 ± 36b | 551 ± 65A | 0.97 | AD | 611 ± 49c | 561 ± 45A | CI<1.0 | S |
| Insoluble β-glucan + Frankincense EO | 93 ± 6a | 1282 ± 76B | 1.75 | A | 337 ± 54a,b | 756 ± 122A | 1.06 | AD | 796 ± 279a,b,c | 448 ± 157A | CI<1.0 | S |
| ‡The highest concentration of insoluble glucan and Sacred EO were selected for combination treatments against CHO-K1 cells, as no IC50 values were observed when tested separately. EO: Essential oil. IC50: Concentration that inhibits 50% of the cellular growth. CI: Combination Index. CE: Combinatory effect. NR: Not reached. NA: Not applicable. Additive effect (AD): CI ≈ 1.0. Antagonistic effect (A): CI>1.0. Synergistic effect (S): CI<1.0. Results are presented as average ± standard deviation of at least 3 independent experiments. IC50 values of insoluble glucan bearing different lowercase letters are significantly different (p ≤ 0.05). IC50 values of total EOs bearing different uppercase letters are significantly different (p ≤ 0.05). |
||||||||||||
Table 1: Effect of EOs used separately and in combination with insoluble β-glucan on the induction of QR.
Effect of EOs in combination with insoluble β-glucan on the cellular proliferation of different cell lines
The effect of B. carterii (Frankincense) and B. sacra (Sacred) EOs on the growth inhibition of human CRC HT-29 and CaCO2 cells as well as on non-cancerous Cho-K1cells were evaluated and the results are presented in Table 2. Regarding HT-29 cells, IC50 values of 1447 ppm and 1348 ppm were found for B. carterii (Frankincense) and B. sacra (Sacred) EOs respectively. Against CaCO2 cells, IC50 values of 1424 ppm and 1138 ppm were found for B. carterii (Frankincense) and B. sacra (Sacred) EOs respectively. Concerning the non-cancerous CHO-K1 cells, B. carterii (Frankincense) EO exhibited an IC50 value of 1689 ppm whereas B. sacra (Sacred) EO showed no effect on this noncancerous cell line at tested concentrations ranging from 21.5 to 2752 ppm. The results hence suggest that B. sacra (Sacred) EO exhibited cancerous cell-specific cytotoxicity. In addition, no significant difference between IC50 values of B. carterii (Frankincense) and B. sacra (Sacred) EOs tested separately were observed regarding HT-29 and CaCO2 cell lines (P>0.05).
| Samples* | Concentration (ppm) | Fold Induction | Effect | |
|---|---|---|---|---|
| Insoluble glucan | Total EOs | |||
| Control | NA | NA | 0.8 ± 0.4b | NA |
| Sacred EO | NA | 54 | 1.4 ± 0.1c | NA |
| Frankincense EO | NA | 27 | 1.5 ± 0.1c | NA |
| Insoluble glucan | 250 | NA | 1.5 ± 0.2c | NA |
| Frankincense EO + Sacred EO † | NA | 81 | 1.4 ± 0.2c | I |
| Insoluble glucan + Sacred EO | 250 | 54 | 0.4 ± 0.2a | A |
| Insoluble glucan + Frankincense EO | 250 | 27 | 2.0 ± 0.5d | AD |
| *Concentrations of essential oil (EO) tested separately ranged from 3 to 1720 ppm. †: 27 ppm of Frankincense and 54 ppm of Sacred EOs were combined. Additive effect (AD): 1.5 <Fold induction >3.0. No interactive effect (I): Fold induction ≈ 1.5. Antagonistic effect (A): 1.5 >Fold induction. NA: Not applicable. Concentrations for combined treatments were chosen based on the highest induction of each compounds tested separately. Means followed by different letters are significantly different (p ≤ 0.05). Results are presented as average ± standard deviation of at least 3 independent experiments. |
||||
Table 2: Effect of EOs used separately and in combination with insoluble β-glucan on the cellular proliferation of different cell lines.
In order to increase the chemo-preventive potential of B. carterii (Frankincense) and B. sacra (Sacred) EOs, these EOs and insoluble β-glucan were used in combination to evaluate their effect on the growth of different cell lines. Table 2 indicates that the combination of B. carterii (Frankincense) and B. sacra (Sacred) EOs against HT-29 cells exhibited a combination index (CI) of 1.05 and an IC50 value in total EOs of 1465 ppm, thus suggesting an additive effect. In contrast, B. carterii (Frankincense) and B. sacra (Sacred) EOs in combination with insoluble β-glucan (which showed an IC50 value of 108 ppm when tested separately) presented a CI values of 2.61 and 1.75 respectively. However, combined treatments revealed that IC50 values of insoluble β-glucan (93 ppm for both EOs) and EOs (1279 and 1282 ppm respectively) were not significantly different from IC50 values obtained when tested separately (P>0.05). Those results suggest that EOs combined with insoluble β-glucan generated antagonistic effects against HT-29 cells.
In the case of CaCO2 cells, combination of B. carterii (Frankincense) and B. sacra (Sacred) EOs exhibited a CI value of 0.82 and an IC50 value in total EOs of 1032 ppm, hence suggesting an additive effect which is congruent with results observed with HT-29 cells. However, B. carterii (Frankincense) and B. sacra (Sacred) EOs in combination with insoluble β-glucan (which showed an IC50 value of 634 ppm when tested separately) presented CI values of 0.97 and 1.06 respectively. However, B. carterii (Frankincense) and B. sacra (Sacred) EOs in combination with insoluble β-glucan showed that IC50 values of insoluble β-glucan (307 and 337 ppm respectively) and EOs (551 and 756 ppm respectively) are lower than IC50 values obtained when tested separately. Those results suggest that EOs combined with insoluble β-glucan demonstrated additive effects against CaCO2 cells.
Table 2 also indicates that the combination of B. carterii (Frankincense) and B. sacra (Sacred) EOs against CHO-K1 cells exhibited an IC50 value in total EOs of 704 ppm, hence suggesting a synergistic effect. Moreover, B. carterii (Frankincense) and B. sacra (Sacred) EOs in combination with insoluble βglucan revealed IC50 values of insoluble β-glucan (611 and 796 ppm respectively) and EOs (561 and 448 ppm respectively), hence suggesting a synergistic effect since insoluble β-glucan and B. sacra (Sacred) EOs assayed separately showed no IC50 values against CHO-K1 at tested concentrations ranging from 21.5 to 2752 ppm. These results suggest that CHO-K1 cells revealed to be highly sensitive to a combination of insoluble β-glucan and EOs, possibly due to an important cytotoxic effect on noncancerous cells and the loss of cancerous cell-specific cytotoxicity.
Effect of EOs on the level of apoptosis in human colorectal cancer cells
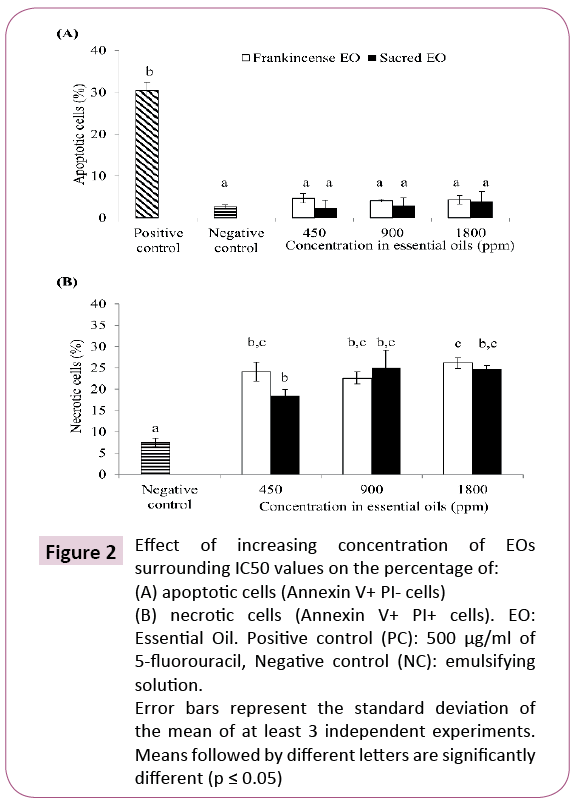
Since a cancerous cell-specific cytotoxicity was observed with B. sacra (Sacred) EO tested separately, Annexin V-FITC/PI double staining was performed to determine if apoptosis was involved in the growth inhibition of cancerous cells by EOs. HT-29 cells were chosen for this test since IC50 values of B. carterii (Frankincense) and B. sacra (Sacred) EOs were not significantly (P>0∙05) different against HT29 and CaCO2 cell lines (Table 2). Further, the effect of B. carterii (Frankincense) and B. sacra (Sacred) EOs on apoptosis induction in HT-29 cells has never been investigated previously. Data shown in Figure 2A demonstrated that at the tested concentrations (450, 900 and 1800 ppm), neither B. sacra (Sacred) EO (percentage of apoptotic cells of 2.38%, 2.96% and 3.90%, respectively) nor B. carterii (Frankincense) EO (percentage of apoptotic cells of 4.70%, 4.06% and 4.34%, respectively) were able to significantly (P>0∙05) induce apoptosis (Annexin V + PI - cells) in HT-29 cells at concentrations surrounding IC50 values compared to the negative control (percentage of apoptotic cells of 2.61%). In contrast, data shown in Figure 2B demonstrated that at the tested concentrations (450, 900 and 1800 ppm), B. sacra (Sacred) EO (percentage of apoptotic cells of 18.43%, 25.02% and 24.76%, respectively) and B. carterii (Frankincense) EO (percentage of apoptotic cells of 24.11%, 22.62% and 26.17%, respectively) were able to significantly (P ≤ 0∙05) increase the percentage of necrotic cells as compared to negative control (percentage of necrosis cells of 7.57%) which corresponds to annexin V + PI + cells. Those results suggest that B. carterii (Frankincense) and B. sacra (Sacred) EOs induced cytotoxicity in HT-29 cells via necrosis rather than apoptosis, based on the analysis of externalization of phosphatidylserine on the surface of the cell membrane using Annexin V-FITC and PI double staining.
Figure 2: Effect of increasing concentration of EOs surrounding IC50 values on the percentage of: (A) apoptotic cells (Annexin V+ PI- cells) (B) necrotic cells (Annexin V+ PI+ cells). EO: Essential Oil. Positive control (PC): 500 μg/ml of 5-fluorouracil, Negative control (NC): emulsifying solution. Error bars represent the standard deviation of the mean of at least 3 independent experiments. Means followed by different letters are significantly different (p ≤ 0.05)
Discussion
Evaluation of the antiradical properties of EOs
Antiradical assays revealed that B. sacra (Sacred) EO were more efficient than B. carterii (Frankincense) EO to scavenge O2- anion. This fact underlies the differences between biological activities between them even if both EOs were ineffective to scavenge DPPH radical. Many studies reported the weak capacity of B. carterii (Frankincense) and B. sacra (Sacred) EOs to scavenge DPPH radical, which is in congruence with our results [28,29]. For instance, it was found that B. sacra EO reached 8% scavenging activity at 1000 ppm [28]. Moreover, it was reported that an EO from B. carterii scavenged 50% of DPPH radical at 15210 ppm (15.21 mg/ml), which is at very high concentration [29]. In addition, it was reported that an EO from B. sacra exhibited a greater capacity to scavenge O2- anion (56.40%) as compared to DPPH radical (16.30%) at tested concentrations, similarly to results obtained in the present study [30]. This difference in capacity to scavenge O2- anion more efficiently than DPPH radical can be explained by the lower reactivity of DPPH radical compared to reactive oxygen species (ROS) such as O2- anion [31- 33]. Likewise, it is generally accepted that ROS, especially the O2- anion, are the most important free radicals in many diseases including cancer [34,35]. Such O2- anion scavenging activity of B. sacra (Sacred) EO may be due to its high content of terpene as compared to B. carterii (Frankincense) EO, such as α-pinene, as reported [9,36]. It will be necessary to quantify the exact content and nature of terpenes found in both EO to verify this hypothesis.
Effect of EOs in combination with insoluble β-glucan on the induction of NAD(P)H: QR and Mw determination
The results of QR assays results showed that B. carterii (Frankincense) and B. sacra (Sacred) EOs were able to induce QR activity at low concentrations (54 and 26 ppm respectively). An increase of the QR specific activity byBoswellia spp. EOs has never been reported until now, hence revealing a novel chemo-preventive property of these EOs. Thus, mechanisms by which EOs fromBoswellia spp. induce QR are worthy of further discussion. The increase of gene transcription coding for phase II enzymes depends on the destabilization of Keap1/Nrf cytoplasmic complex which triggers antioxidant response element (ARE) release. This destabilization is related to an α,β-unsaturated ketone moiety of an inducer reacting with the cysteine thiol of Keap1 [37-39]. Unsaturated ketones (enones) are known to be present in EOs fromBoswellia spp. Indeed, traces of rotundone and mustakone, two sesquiterpene ketones (aromatic enones), in B. sacra (Sacred) EO were detected [40].
Moreover,Boswellia spp. EOs contains keto-β-boswellic acid (KBA) and acetyl-keto-β-boswellic acid (AK-BA) which also possess aromatic enone functional groups [41]. Thus, such molecules in B. carterii (Frankincense) and B. sacra (Sacred) EOs might explain the increase of QR activity observed in the present study especially since other organic acids and triterpenoid compounds were proven to be QR inducers such as fumaric acid derivatives, coussaric acid A and bardoxolone methyl [42-45].
In order to increase the chemo-preventive potential of B. carterii (Frankincense) and B. sacra (Sacred) EOs, they were combined with insoluble β-glucan of S. boulardii’s cell wall. In previous studies, this insoluble β-glucan showed the most relevant chemopreventive properties in vitro and in vivo [17,18]. The combination of insoluble β-glucan andBoswellia spp. EOs as potential chemopreventive agents has never been tested before. Our results showed that insoluble β-glucan enhanced QR activity induced by B. carterii (Frankincense) EO whereas a similar was not observed on B. sacra (Sacred) EO. The degradation of β-glucan by B. sacra (Sacred) EO was not found to be responsible for the observed differences between EOs since the Mw of insoluble β-glucan was similar despite mixing with B. sacra (Sacred) EO. Moreover, many studies demonstrated that EOs may be encapsulated in polysaccharide based gels without negatively affecting their properties [46,47], which rejects the hypothesis of insoluble β-glucan degradation. However, divergence of combinatory effect between EOs regarding the QR assay may be due to an increased sensitivity of Hepa 1c1c7 cells toward B. sacra (Sacred) EO leading to a weak induction. Thus, future studies on this aspect are necessary.
Effect of EOs in combination with insoluble β-glucan on the cellular proliferation of different cell lines
Many studies demonstrated thatBoswellia spp. EOs exhibit cytotoxic effects (antiproliferative activity) toward different cancerous cell lines. It was demonstrated that an EO from B. sacra exhibited IC50 values varying from 1:1680 (1264 ppm) to 1:1800 (477 ppm) toward human breast cancer cells [41]. Moreover,4 EO fractions from B. sacra exhibiting IC50 values varying from 1:270 (3185 ppm) to 1:1560 (551 ppm) toward human pancreatic cancer cells was obtained [48].
Finally, it was demonstrated that an EO from B. carterii exhibited an IC50 value of 1:1250 (688 ppm) toward human bladder cancer cells whereas in another research an IC50 value of 1:600 (1433 ppm) against the same cell line was obtained [6,7]. These investigations confirm the congruence of IC50 values obtained in the present study with the scientific literature.
It is largely accepted that B. carterii is merely a synonym for B. sacra. However, significant differences were observed in the composition of EOs obtained from these both plants [9]. The authors reported that B. sacra (Sacred) EO differed from B. carterii (Frankincense) EO on higher optical rotation values (+30.1 and -13.3° respectively), enantiomeric ratios values and α-pinene content (79.0 and 48.2% respectively), which confirmed that both species are distinct. Terpenes contained inBoswellia spp. EOs are known to influence cancerous cell-specific cytotoxicity. Indeed 2 EOs from B. sacra were extracted and observed that EOs with higher boswellic acid (BA) content exhibited higher cancerous cell-specific cytotoxicity in breast cancer cells. EO from B. sacra was extracted containing a high concentration of α-pinene (62%) and α-amyrin (21%) which exhibited a more efficient antiproliferative effect on a human breast cancer cell line as compared to a similar EO containing less α-amyrin, hence suggesting a combined effect of terpenes [41,49]. Moreover, enantiomeric ratios can influence biological activities of EOs. Indeed, it was demonstrated that α and β-(+)-pinenes, which are the most abundant terpenes inBoswellia spp. [50]. EOs, exhibited minimal inhibitory concentration (MIC) values against Candida albicans, Cryptococcus neoformans, Rhizopus oryzae and Methicillin-resistant Staphylococcus aureus whereas α and β-(-) pinenes showed no effect at tested concentrations. Those investigations might explain the divergence in biological activities between EOs observed in the present study notably regarding cancerous cells specific cytotoxicity of B. sacra (Sacred) EO.
Combined treatments demonstrated that the effect of EOs and insoluble β-glucan had different effects on the viability of CaCO2 and HT-29 cell lines whereas no difference (P>0.05) was observed when tested separately. Since no study has investigated the combined effect of EOs and insoluble β-glucan on cell viability, it may be hypothesized that combining these compounds might create different chemical species that acted differently on cell lines. Since it was confirmed that EOs did not affect the Mw of insoluble β-glucan, further investigation will be necessary to understand such an effect. Finally, results showed that all combinations exhibited IC50 values toward CHO-K1 cells, hence suggesting that combinations affected the growth of noncancerous cells since insoluble β-glucan and B. sacra (Sacred).
EO exhibited cancerous cell-specific cytotoxicity when tested separately. Such synergistic effects in CHO-K1 cells might be due to multiple mechanisms triggered by combined treatments in non-cancerous cells, which suggests important side effects of these combinations.
Effect of EOs on the level of apoptosis in human colorectal cancer cells
Cytotoxic activities ofBoswellia spp. EOs. are known to be mainly due to pro-apoptotic properties [6,7,41,48]. However, it is interesting that no apoptotic activity was detected in the present study. Such divergence with the scientific literature may be explained by the fact that no study investigated the apoptosis activities of wholeBoswellia spp. EOs (which contains many bioactive compounds) on HT-29 cells. However, it was demonstrated that boswellic acid (BA), keto-β-boswellic acid (KBA) and acetyl-keto-β-boswellic acid (AK-BA) induced apoptosis in a dose-dependent manner in HT-29 cells [51]. Also, it has been reported that 5 μg/ml (5 ppm) of boswellic acid were able to induce phosphatidylserine exposure at the outer membrane of erythrocytes, hence indicating undergoing suicidal erythrocyte death [52]. Those previous studies suggest that B. carterii (Frankincense) and B. sacra (Sacred) EOs used in the present study did not contain sufficient amounts of those specific triterpenes to induce apoptosis in HT-29 cells. Indeed, BA and its derivatives have been frequently reported to correlate with apoptotic activity depending on cell lines and concentrations used. Also, researchers have measured BA content of two EOs from B. sacra obtained at different temperatures and investigated their apoptotic activity in human breast cancer cells [41]. Extracts obtained at 100°C exhibited the highest content in BA (30.1 mg/ml) and showed the most relevant results regarding DNA fragmentation, caspase activation and cell cycle arrest as compared to EOs obtained at 78°C (19.6 mg/ml boswellic acid). Moreover, researchers have obtained 4 EO fractions from B. sacra gum resins and reported that fractions (III and IV) containing high content in BA exhibited apoptosis activity in four different human pancreatic cancer cells [48]. The authors also noticed that expression patterns in time function of pAkt, cdk4 and cyclin D1 proteins differ among those human pancreatic cancer cell lines using cell cycle arrest assay upon treatment with fractions III and IV. More recently, research studies have shown to have treated several cancer cell lines with acetyl-lupeolic acid obtained from the resin of B. carterii, and have assessed the capacity of this compound to inhibit cellular growth and induce apoptosis [53]. The authors noticed important variability among IC50 values obtained. More importantly, they have observed that apoptosis was not induced in the only cell line resistant to the acetyl-lupeolic acid treatment (i.e., no IC50 value obtained). Those studies revealed that induction of apoptosis by EOs fromBoswellia spp. may vary upon terpene composition and cancerous cell lines.
References
- Canadian Cancer Society’s Advisory, Committee on Cancer Statistics, Canadian Cancer Statistics 2015 (2014) Canadian Cancer Statistics 2014. Society CC, Toronto, ON, p. 132.Available from: URL:https://www.colorectalcancer.ca/IMG/pdf/Canadian-Cancer-Statistics-2014-EN.pdf
- Canadian Digestive Health Foundation (2016) Colon Cancer Overview. Consulted on: April 20th 2016.Available from: URL: https://www.cdhf.ca/en/disorders/details/id/7
- Czadek P (2016) Chemoprevention of colorectal cancer. Polish Annals of Medicine 23: 75-79.
- Jaya Kumar S, Madan Kumar A, Ashok Kumar S, Raghunandha Kumar S, GokulaDhas K, et al. (2012) Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem 360: 51-60.
- Gautam N, Mantha AK, Mittal S (2014) Essential oils and their constituents as anticancer agents: a mechanistic view. Biomed Res Int 2014: 154106.
- Frank MB, Yang Q, Osban J, Azzarello JT, Saban MR, et al. (2009) Frankincense oil derived from Boswelliacarteri induces tumor cell specific cytotoxicity. BMC Complement Altern Med 9: 6.
- Dozmorov MG, Yang Q, Wu W, Wren J, Suhail MM, et al. (2014) Differential effects of selective frankincense (Ru Xiang) essential oil versus non-selective sandalwood (Tan Xiang) essential oil on cultured bladder cancer cells: A microarray and bioinformatics study. Chin Med 9: 18
- Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils - A review. Food ChemToxicol 46: 446-475.
- Woolley CL, Suhail MM, Smith BL, Boren KE, Taylor LC, et al. (2012) Chemical differentiation of Boswellia sacra and Boswelliacarteriiessential oils by gas chromatography and chiral gas chromatography-mass spectrometry. J Chromatogr A 1261: 158-163.
- Klis FM, Boorsma A, De Groot PW (2006) Cell wall construction in Saccharomyces cerevisiae. Yeast 23: 185-202.
- Samuelsen AB, Schrezenmeir J, Knutsen SH (2014) Effects of orally administered yeast-derived betaglucans: a review. MolNutr Food Res 58: 183-193.
- Suphantharika M, Khunrae P, Thanardkit P, Verduyn C (2003) Preparation of spent brewer's yeast betaglucans with a potential application as an immuno-stimulant for black tiger shrimp, Penaeusmonodon. BioresourTechnol 88: 55-60.
- Watanabe T, Shimada R, Matsuyama A, Yuasa M, Sawamura H, et al. (2013) Anti-tumor activity of the beta-glucanparamylon from Euglena against pre-neoplastic colonic aberrant crypt foci in mice. Food Funct 4: 1685-1690.
- Aguilar-Uscanga B, Francois JM (2003) A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. LettApplMicrobiol 37: 268-274
- Pinto M, Coelho E, Nunes A, Brandão T, CoimbraMA (2014) Valuation of brewers spent yeast polysaccharides: A structural characterization approach. CarbohydrPolym116: 215-222.
- Ahmad A, Anjum FM, Zahoor T, Nawaz H, Dilshad SM (2012) Beta glucan: A valuable functional ingredient in foods. Crit Rev Food SciNutr 52: 201-212.
- Fortin O, Aguilar-Uscanga B, Vu KD, Salmieri S, Lacroix M (2017) Effect of β-glucan and mannoprotein extracted from cell wall of Saccharomyces boulardii on colon cancer prevention in male F344 rats treated with 1,2-dimethylhydrazine. NutrCancer Submitted.
- Fortin O, Aguilar-Uscanga B, Vu KD, Salmieri S, Lacroix M (2017) Cancer chemopreventive, anti-proliferative and superoxide anion scavenging properties of Kluyveromycesmarxianus and Saccharomyces cerevisiae var. boulardiicell wall components. Nutr Cancer.[In press].
- Gerhäuser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, et al. (2003) Mechanism-based in vitro screening of potential cancer chemo-preventive agents. Mutation Research 523-524
- Blois MS (1958) Anti-oxidant determinations by the use of a stable free radical. Nature 181: 1199-1200.
- Kedare SB, Singh RP (2011) Genesis and development of DPPH method of antioxidant assay. J Food SciTechnol 48: 412-222.
- Megdiche-Ksouri W, Trabelsi N, Mkadmini K, Bourgou S, Noumi A, et al. (2015) Artemisia campestris phenolic compounds have antioxidant and antimicrobial activity. Industrial Crops and Products 63: 104-113.
- Prochaska HJ, Santamaria AB (1988) Direct measurement of NAD(P)H: Quinone reductase from cells cultured in microtiter wells: A screening assay for anti-carcinogenic enzyme inducers. Anal Biochem 169: 328-336.
- Talalay P (1989) Mechanisms of induction of enzymes that protect against chemical carcinogenesis. Adv Enzyme Regul 28: 237-50.
- Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, et al. (1991) Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res 51: 2515-2520.
- Hossain F, Follett P, Dang Vu K, Harich M, Salmieri S, et al. (2016) Evidence for synergistic activity of plantderived essential oils against fungal pathogens of food. Food Microbiology 53: 24-30.
- Berenbaum MC (1977) Synergy, additivism and antagonism in immunosuppression. A critical review. ClinExpImmunol 28: 1-18.
- Ali SK, Hamed AR, Soltan MM, Hegazy UM, Elgorashi EE, et al. (2013) In-vitro evaluation of selected Egyptian traditional herbal medicines for treatment of alzheimer disease. BMC Complementary and Alternative Medicine 13: 1-10.
- Mohamed AA, Ali SI, Kabiel HF, Hegazy AK, Kord MA, et al. (2015) Assessment of antioxidant and antimicrobial activities of essential oil and extracts of Boswelliacarteri resin. International J Pharmacog Phytochemical Res 7: 502-509.
- Al-Harrasi A, Ali L, Ceniviva E, Al-Rawahi A, Hussain J, et al. (2013) Antiglycation and antioxidant activities and HPTLC analysis of Boswellia sacra Oleogum resin: The sacred frankincense. Tropical J Pharm Res 12: 597-602.
- Dizhbite T, Telysheva G, Jurkjane V, Viesturs U (2004) Characterization of the radical scavenging activity of lignins––natural antioxidants. BioresourceTechnol 95: 309-317.
- Letelier ME, Molina-Berrios A, Cortes-Troncoso J, Jara-Sandoval J, Holst M, et al. (2008) DPPH and oxygen free radicals as pro-oxidant of biomolecules. ToxicolIn Vitro 22: 279-286.
- Tai A, Iomori A, Ito H (2017) Structural evidence for the DPPH radical-scavenging mechanism of 2-O-alphad-glucopyranosyl-l-ascorbic acid. Bioorg Med Chem 25:5303-5310.
- Gao X, Schottker B (2017) Reduction–oxidation pathways involved in cancer development: A systematic review of literature reviews. Oncotarget 8: 51888–51906.
- Gholamian-Dehkordi N, Luther T, Asadi-Samani M, Mahmoudian-Sani MR (2017) An overview on natural antioxidants for oxidative stress reduction in cancers; a systematic review. ImmunopathologiaPersa 3: e12.
- Singh HP, Mittal S, Kaur S, Batish DR, Kohli RK (2009) Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J Agric Food Chem 57: 6962-6966.
- Yang Y, Wang Y, Wang T, Jiang X (2017) O26 Screening active components of modified xiaoyao powder for chemoprevention in breast cancer cells: Involvement of the NRF2/NQO1 signalling pathway. BiochemPharmacol 139: 117-118.
- Dinkova-Kostova AT, Kostov RV, Canning P (2017) Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch BiochemBiophys 617:84-93.
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, et al. (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. ProcNatlAcadSci U S A 99: 11908-11913.
- Niebler J, Zhuravlova K, Minceva M, Buettner A (2016) Fragrantsesquiterpene ketones as trace constituents in frankincense volatile oil of Boswellia sacra. JNatural Products 79: 1160-1164.
- Suhail MM, Wu W, Cao A, Mondalek FG, Fung KM, et al. (2011) Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement Altern Med 11: 129.
- Kang YH, Pezzuto JM (2004) Induction of quinonereductase as a primary screen for natural product anticarcinogens. Methods Enzymol 382: 380-414.
- Spencer SR, Wilczak CA, Talalay P (1990) Induction of glutathione transferases and NAD(P)H: Quinone reductase by fumaric acid derivatives in rodent cells and tissues. Cancer Res 50: 7871-7875
- Wang YY, Yang YX, Zhe H, He ZX, Zhou SF (2014) Bardoxolone methyl (CDDO-Me) as a therapeutic agent: An update on its pharmacokinetic and pharmacodynamic properties. Drug Des DevelTher 8: 2075-2088.
- Dai H, Jiao Q, Liu T, You Q, Jiang Z (2017) Development of novel Nrf2/ARE inducers bearing pyrazino[2,1a]isoquinolinscaffold with potent in vitro efficacy andenhanced physicochemical properties. Molecules 22.
- Beyki M, Zhaveh S, Khalili ST, Rahmani-Cherati T, Abollahi A, et al. (2014) Encapsulation of Menthapiperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillusflavus. Industrial Crops and Products 54: 310-319.
- Ahmed TA, Aljaeid BM (2016) Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des DevelTher 10: 483-507.
- Ni X, Suhail MM, Yang Q, Cao A, Fung KM, et al. (2012) Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complement Altern Med 12: 253.
- Hakkim FL, Al-Buloshi M, Al-Sabahi J (2015) Frankincense derived heavy terpene cocktail boosting breast cancer cell (MDA-MB-231) death in vitro. Asian Pacific Journal of Tropical Biomedicine 5: 824-828.
- Rivas Da-Silva AC, Lopes PM, Barros de Azevedo MM, Costa DC, Alviano CS, et al. (2012) Biological activities of alpha-pinene and beta-pinene enantiomers. Molecules 17, 6305-2316.
- Liu JJ, Nilsson A, Oredsson S, Badmaev V, Zhao WZ, et al. (2002) Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis 23: 2087-2093.
- Calabrò S, Alzoubi K, Faggio C, Laufer S, Lang F (2015) Triggering of suicidal erythrocyte death following boswellic acid exposure. CellularPhysiolBiochem 37: 131-142.
- Schmidt C, Loos C, Jin L, Schmiech M, Schmidt CQ, et al. (2017) Acetyl-lupeolic acid inhibits Aktsignaling and induces apoptosis in chemoresistant prostate cancer cells in vitro and in vivo. Oncotarget 8: 55147-55161.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences