A Three Strain Postbiotic Mix Improves the Immune System Function after Oral Intake for 28-Days
Jordi Cune Castellana, Maria Tintore Gazulla, Justyna Meissner, Marian Merino, Jose Luis Mullor and Carlos de Lecea Flores de Lemus
AB Biotek Human Nutrition & Health, Hampton Peterborough, England Bionos Biotech SL, Biopolo Hospital La Fe, Valencia, Spain
Published Date: 2024-02-19DOI10.36648/ipctn.9.1.39
Jordi Cuñé Castellana1*, Maria Tintoré Gazulla1, Justyna Meissner2, Marián Merino2, José Luis Mullor2 and Carlos de Lecea Flores de Lemus1
1AB Biotek Human Nutrition & Health, Peterborough, United Kingdom
2Bionos Biotech SL, LabAnalysis group, Biopolo Hospital La Fe, Valencia, Spain
- *Corresponding Author:
- Jordi Cuñé Castellana
AB Biotek Human Nutrition & Health,
Hampton Peterborough,
England,
Email: Jordi.Cune@abbiotekhealth.com
Received date: January 19, 2024, Manuscript No. IPCTN-24-18564; Editor assigned date: January 22, 2024, PreQC No. IPCTN-24-18564 (PQ); Reviewed date: February 05, 2024, QC No. IPCTN-24-18564; Revised date: February 12, 2024, Manuscript No. IPCTN-24-18564 (R); Published date: February 19, 2024, DOI: 10.36648/ipctn.9.1.39
Citation: Castellana JC, Gazulla MT, Meissner J, Merino M, Mullor JL, et al. (2024) A Three Strain Postbiotic Mix Improves the Immune System Function after Oral Intake for 28-Days. J Nutraceuticals Food Sci Vol.9 No.1: 39.
Abstract
Background: Dietary selenium complements are known to improve overall health and enhance different aspects of the immune response, participating in the function of the immune cells, cytokine secretion, regulation of chronic inflammation and control of excessive immune responses. In this study, our objective was to evaluate whether a selenium-rich postbiotic, composed of whole-cell Saccharomyces cerevisiae ABB S15, Kluyveromyces marxianus ABB S8 and Saccharomyces boulardii ABB S3 yeast strains, can act as provider of selenium to enhance the function of the human immune system after oral intake.
Methods and findings: A total of 15 volunteers were enrolled in a 28-day clinical study in which they complemented their normal diets with a daily dose of a novel selenium food supplement, “postbiotic immune”. Selenium levels in plasma were quantified by ELISA (Enzyme Linked Immuno Sorbent Assay), and the proliferation and activation of different subsets of lymphocytes isolated from PBMC’s (Peripheral Blood Mononuclear Cells) were assessed by flow cytometry using different cell markers. At the end of the study, we observed a higher proliferative capacity of total lymphocytes and specifically, of CD16+ lymphocytes (natural killer cells), upon stimulation with the CD3 and CD28 antibodies mimicking cell activation in vitro. Interestingly, we did not observe an increase in the activation of the lymphocyte subsets CD4+ (T helper cells) but CD16+, indicating that there was a specific activation of natural killer cells.
Conclusions: Altogether, our results suggest that orally ingested “Postbiotic immune” primes the human immune system to elicit a faster and more efficient immune response through the specific activation of natural killer cells.
Keywords
Selenium; Food; Supplement; Postbiotic; Plasma; Immune system; Lymphocyte; Human; T helper cells; Natural killer cells
Introduction
Selenium (Se) is an essential micronutrient involved in multiple aspects of human health including proper thyroid hormone metabolism, cardiovascular health, prevention of neurodegeneration and cancer, and optimal immune responses [1]. Dietary selenium is incorporated into selenoproteins, which are proteins containing selenium in the form of the 21st amino acid, selenocysteinen, that participate in a variety of vital physiological processes, including inflammation and immunity [2]. Most of the selenium in the body is stored in the muscle tissue, although the highest concentration of selenium is found in the thyroid gland due to various selenoproteins that participate in the thyroid function [3].
To date, many studies have been conducted on the benefits of selenium intake in the enhancement of the function of the immune system. Appropriate levels of selenium are important for triggering a well-orchestrated immune response, participating in the function of the immune cells, cytokine secretion, regulation of chronic inflammation and excessive immune responses [4]. Importantly, selenium deficiency has a negative effect on activation, differentiation, and proliferation of immune cells, due to the excessive oxidative stress and defective protein folding and calcium transport [4]. In oncology, it has been shown that selenium supplementation counters the immunosuppression associated to tumor microenvironments towards antitumor immunity by activating immune cells (e.g. M1 macrophages and CD8+ T-lymphocytes) and producing proinflammatory cytokines such as interferon-gamma [5,6].
In dietary supplementation, the use of organic selenium is preferred instead of inorganic selenium, since organic compounds are more easily absorbed by human organisms in comparison with inorganic compounds [7]. In this regard, selenium enriched postbiotics are a common source of organic selenium used to supplement the dietary intake of this important trace mineral, and their use has been approved for human consumption in the European Union and Britain [7] (EFSA (European Food Safety Authority) positive list of QPS species (Qualified Presumption of Safety)) [8]. Although the use of oral probiotics is widespread, there are some concerns regarding the use of live probiotics, such as cases of systemic infections, acquisition of antibiotic resistance genes or interference with gut colonization in neonates [9]. To avoid these risks, there is an increasing interest in using postbiotics, preparations of inanimate microorganisms and/or their components that confer a health benefit on the host [10]. An example of postbiotics are heat killed, non-viable microorganisms or microbial cell extracts, which have a number of beneficial effects [11]. For example, heat killed Saccharomyces cerevisiae has shown to improve the immune response when used as vaccine in mice, while heat killed cells of Saccharomyces boulardii maintain intestinal integrity, modulate the immune system and prevent bacterial translocation and intestinal lesions in mice [12,13]. There is evidence supporting that S. boulardii has the ability to stimulate he host immune system by triggering the release of immunoglobulins [14]. Interestingly, the yeast Kluyveromyces marxianus has also many beneficial effects on human health, such as inducing apoptosis in different human cancer cells, controlling intestinal inflammation and cellular oxidative stress, and modulating the immune response by reducing proinflammatory cytokines in the presence of inflammatory stimulus, therefore its use as postbiotic could also be beneficial for the human organism [15,16].
In the human immune system, T cells are particularly important due to their role in promoting adaptive immunity against pathogens and cancer as well as regulating tolerance, all of which are influenced by dietary selenium levels. Selenoproteins exhibit a wide variety of functions within T cells that include regulating calcium flux induced by T Cell Receptor (TCR) engagement, shaping the redox state of T cells before during and after activation and linking TCR induced activation to metabolic reprogramming required for T cell proliferation and differentiation [17]. Specifically, selenium supplementation has been reported to influence the activity of two important immune cells; T helper lymphocytes and Natural Killer (NK) cells. T helper cells (CD4+, CD8-) are a heterogeneous cell population comprising different subsets that exert distinct roles in cellmediated immunity. CD40 is expressed on the surface of numerous immune and non-immune cells, whereas CD40L is expressed primarily by activated CD4+ T cells [18]. It has been shown that a higher reductive state induced through increased dietary selenium intake favors T helper cells differentiation into Th1 subset during the proliferation and activation of naive CD4+ T cells [19]. Th1 cells stimulate cellular immune response whilst Th2 subset stimulates humoral immune response promotes B cell proliferation and antibody production [20]. Selenium supplements augmented the cellular immune response through an increased production of interferon γ and other cytokines, an earlier peak T cell proliferation, and an increase in T helper cells whilst humoral immune responses were unaffected [21]. On the other hand, NK cells are important players of the innate immune system that destroy infected and diseased cells. Activated NK cells are characterized by the marker CD107a, which is an indicator of the NK cell functional activity CD16+/CD107a+ [22]. Interestingly, selenium supplementation has been reported to enhance the activation and lytic activity of NK cells [23]. Overall, it is well established that optimal selenium levels are associated with enhanced T cell proliferation, activation of T helper cells, increase NK cell count and activity, stronger vaccine responses and robust immunity against pathogens. [24].
The goal of this study was to evaluate the effect of a selenium rich postbiotic, “Postbiotic immune”, a combination of whole cell S. cerevisiae ABB S15, K. marxianus ABB S8 and S. boulardii ABB S3, with high content in organic selenium, in the improvement of the function of the immune system in human volunteers. This was assessed by measuring different parameters in the peripheral blood mononuclear cells isolated from volunteers’ blood, such as the selenium bioavailability in the plasma of volunteers and the proliferation and activation of specific lymphocyte subsets by flow cytometry (total lymphocytes and CD4+, CD4+/CD40L+, CD16+, CD16+/CD107a+ lymphocytes). Altogether, our results suggest that oral intake of “Postbiotic immune” for 28 days primes the human immune system to elicit a faster and more efficient immune response by specifically activating the of natural killer cells subpopulation.
Materials and Methods
Tested product
The investigational product “Postbiotic immune” was composed of a synergistic combination of different selenium enriched yeast species; Saccharomyces cerevisiae, Saccharomyces boulardii and Kluyveromyces marxianus. These yeast species are included in the EFSA positive list of QPS species [8].
Study population
The study protocol was approved by the ethical committee for medical research from hospital La Fe, Valencia, Spain (Registration number: 2023-363-1; title: “Clinical evaluation of the improvement of the immune system function after oral intake by quantifying specific biomarkers in the blood of human volunteers”). A total of 15 healthy volunteers between 18 and 65 years old, men and women, were enrolled in this clinical study. The information of volunteers is detailed in Table S1. The exclusion criteria for the selection of volunteers were: (i) Subjects who did not give their consent to participate in the study; (ii) Subjects who were enrolled in a diet different from their usual diet at the time of the start of the study; (iii) Subjects who were taking drugs or supplements that could affect the immune system (zinc supplements, selenium supplements, probiotics, postbiotics, etc.); (iv) Subjects that were on an antibiotic treatment within the 4 weeks prior to enrolment in the study; (v) Subjects that had any infectious disease within the last 4 weeks prior the start of the study; (vi) Subjects diagnosed with immune diseases. Additionally, volunteers were asked to keep their personal eating habits and do not take drugs or dietary supplements aimed at reducing weight or regulating gastrointestinal metabolic parameters during the study period.
Subjects did not show any type of gastrointestinal or allergic reaction after intake of the product; therefore, no subjects were withdrawn from the study. Consumption control was carried out to verify that volunteers follow the guidelines and take the treatment, and to evaluate the clinical adherence. Data of the consumption control is shown in Table S2.
Treatment
The product “Postbiotic immune” was provided in capsules for oral intake, and volunteers took one capsule per day for 28 days, preferably early in the morning after breakfast. Then, venous blood samples were obtained from volunteers before (day 0) and at the end of the treatment (day 28) by trained personnel, and the effect of the treatment was evaluated by measuring different parameters in the blood of 15 volunteers.
Selenium quantification in plasma
Venous blood samples from volunteers were collected in EDTA (Ethylene Diamine Tetra Acetic acid) containing tubes (Acefesa EDTA K2 blood extraction tube, #367525) before (day 0) and at the end of the treatment (day 28). Afterwards, samples were centrifuged at 1000 RPM (Revolutions Per Minute) for 5 minutes at room temperature, and plasma was collected in a separate tube. Then, selenium levels in plasma were quantified by using the selenium assay kit (Abcam, #abx298910), following the manufacturer’s instructions.
Flow cytometry
In order to evaluate the effect of “Postbiotic immune” in the improvement of the function of the immune system, we determined the effect of the treatment on the total count of lymphocytes and the activation of specific subsets of lymphocyte by flow cytometry. Blood samples taken from volunteers before (day 0) and at the end of the treatment (day 28) were mixed with PBS+2% Fetal Bovine Serum (FBS, Gibco, #10500-064) and added to a prefilled tube with lymph prep solution (STEMCELL Technologies, #07801). Afterwards, samples were centrifuged at 800 g for 30 min in SepMate™-50 tubes (STEMCELL Technologies, # 85450) and the plasma: Lymphoprep interface containing Peripheral Blood Mononuclear Cells (PBMCs) was collected in a separate tube. The PBMCs were washed, re-suspended in 1 ml RPMI medium 1640 (Gibco, #21875-034) and divided into two aliquots: One aliquot was seeded on 24-well plates (nonactivated cells) and the other aliquot was supplemented with anti-CD3 (Abcam, #16669) and anti-CD28 (Abcam, #243228) antibodies at 3 μg/ml concentration for 24 hours, thus mimicking αβ-T cell receptor (TCR complex) mediated activation (activated cells). Treatment of cells with monoclonal anti-CD3 antibodies and anti-CD28 antibodies triggers a co-stimulatory signal that can be used for cell activation [25-32]. After the 24-hour incubation period, flow cytometry analysis was performed for quantification of total lymphocyte population and for the subsets CD4+, CD4 +CD40L+, CD16+ and CD16+CD107a+ cells by using the following antibodies according to manufacturer’s instructions: Pacific blue mouse anti-human CD4 (BD Biosciences, #558116), APC mouse anti-human CD40L (BD Biosciences, #555702), FITC mouse antihuman CD16 (BD Biosciences, #561308) or PE mouse anti-human CD107a (BD Biosciences, #555801) antibodies for 30 minutes at room temperature. Flow cytometry analysis was set with 50.000 events per sample and used software was BD FACSDiva 8.0.1.
Self-assessment questionnaire
The efficacy of the treatment was subjectively evaluated by the volunteers. For this, a questionnaire was provided to the 15 volunteers and answers were collected at the end of the treatment. For the self-assessment questionnaires, opinions are given according to parameters from 1 to 5, (1=Strongly Disagree, 2=Disagree, 3=Neutral, 4=Agree, 5=Strongly Agree). For positive impressions, satisfaction is considered when volunteers scored parameters from 4 to 5. Detailed results are shown in Tables S3 and S4.
Statistical analysis
Statistical analysis was performed with graph pad prism V8. Data outliers were identified with ROUT method (Q=5%) and excluded from the analysis if found. Data were statistically analyzed by paired T test. Statistical significance was declared at p<0.05, 95% of confidence. Bars in the charts represent the mean value for each condition and error bars indicate the Standard Error of Mean (SEM) for each group of values. The % of total cells quantified by the flow cytometer analysis software is represented.
Results
Quantification of selenium levels in plasma
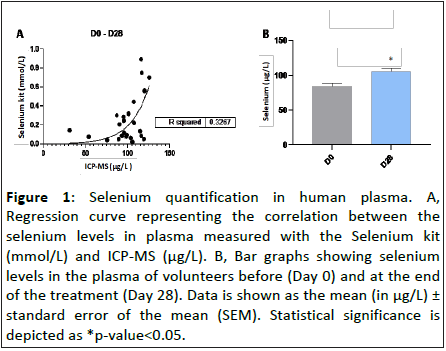
Food supplements containing selenium rich ingredients are commonly used to increase the selenium levels in our organism and to improve the health status of individuals. In order to evaluate whether the oral intake of “Postbiotic immune” for 28 days increased the selenium levels in plasma, we quantified the amount of selenium in the plasma obtained from volunteers before (Day 0) and at the end of the treatment (Day 28). As Figure 1 shows, daily oral intake of “Postbiotic immune” for 28 days resulted in an increase of selenium levels in the plasma of volunteers by 108.7 ± 30.5% compared to day 0 (Figure 1), demonstrating that the product “Postbiotic immune” is a dietary supplement able to increase plasma selenium levels in healthy individuals.
Figure 1: Selenium quantification in human plasma. A, Regression curve representing the correlation between the selenium levels in plasma measured with the Selenium kit (mmol/L) and ICP-MS (μg/L). B, Bar graphs showing selenium levels in the plasma of volunteers before (Day 0) and at the end of the treatment (Day 28). Data is shown as the mean (in μg/L) ± standard error of the mean (SEM). Statistical significance is depicted as *p-value<0.05.
Flow cytometry analysis of lymphocyte subsets
It has been widely shown that selenium diet supplementation has a positive impact on the function of the human immune system. In order to evaluate whether “Postbiotic immune” enhances the function of the human immune system, we determined the proliferation of total lymphocytes, as well as activated and non-activated CD4+ (T-Helper) and CD16+ (Natural killer “NK”) lymphocytes by flow cytometry. For this, we collected the PBMC fraction from the blood of volunteers before (Day 0) and at the end of the treatment (Day 28), and stimulated them with anti-CD3 and anti-CD28 antibodies (including a nonstimulated condition). These antibodies mimic the molecular events occurring during an immune response process by triggering a co-stimulatory signal involving the TCR complex on T cells [25-28]. The CD28, a cell surface molecule expressed on all T cells, acts as a major costimulatory receptor in promoting full activation of naive T cells. CD28 has been shown to activate human NK cells by stimulating cytotoxicity in a process dependent on phosphoinositide-3 kinase activation, leading to sustained Extracellular Signal-Regulated Kinase 2 (ERK2) phosphorylation. CD28-mediated co-stimulation is also necessary for optimal proliferation of NK cells [29-32]. After antibody stimulation, total lymphocytes and specific lymphocyte subsets were quantified as described above.
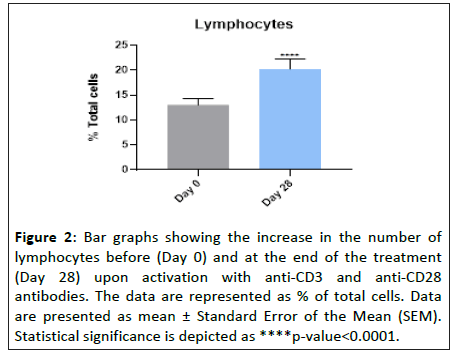
The increase in the total lymphocyte count was calculated by comparing the activated vs the non-activated conditions, and represented for both day 0 and day 28 (Figure 2). Our results showed that daily oral intake of “Postbiotic immune” for 28 days increased the total number of lymphocytes by 55.7 ± 9.6% upon antibody stimulation, when compared to day 0 (Figure 2). This result indicates that “Postbiotic immune” promotes lymphocyte proliferation in an in vitro model of cell activation.
Figure 2: Bar graphs showing the increase in the number of lymphocytes before (Day 0) and at the end of the treatment (Day 28) upon activation with anti-CD3 and anti-CD28 antibodies. The data are represented as % of total cells. Data are presented as mean ± Standard Error of the Mean (SEM). Statistical significance is depicted as ****p-value<0.0001.
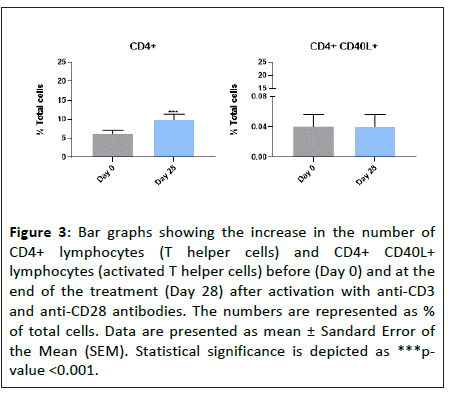
We then analyzed the effect of daily oral intake of “Postbiotic immune” for 28 days on the CD4+ lymphocyte subset (T helper cells). Our data showed that the treatment increased the total number of CD4+ lymphocytes by 59.4 ± 12.6% after antibody stimulation, when compared with day 0 (Figure 3). However, the treatment with “Postbiotic immune” did not increase the number of CD4+ CD40L+ lymphocytes (activated T-helper cells) upon antibody stimulation (Figure 3). These findings evidence that “Postbiotic immune” promotes the proliferation of the CD4+ lymphocyte population in a similar extent than the total lymphocyte population (55.7% vs 59.4%), and that the product does not mediate activation of the CD4+ lymphocytes upon the in vitro activation by the anti-CD3/CD28 antibodies.
Figure 3: Bar graphs showing the increase in the number of CD4+ lymphocytes (T helper cells) and CD4+ CD40L+ lymphocytes (activated T helper cells) before (Day 0) and at the end of the treatment (Day 28) after activation with anti-CD3 and anti-CD28 antibodies. The numbers are represented as % of total cells. Data are presented as mean ± Sandard Error of the Mean (SEM). Statistical significance is depicted as ***pvalue <0.001.
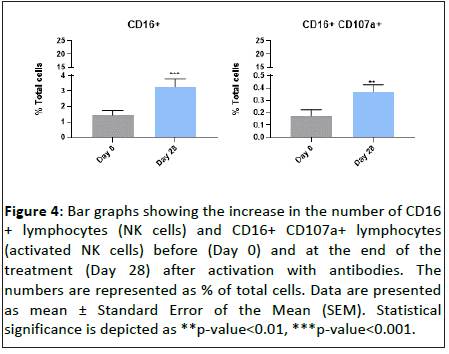
On the other hand, we evaluated how the CD16+ lymphocyte (NK cells) subset responded to the daily oral intake of “Postbiotic immune” for 28 days. Our results demonstrated that the treatment increased the total number of CD16+ lymphocytes by 127.3 ± 29.6% after antibody stimulation, when compared to day 0 (Figure 4). In addition, the 28-day treatment with “Postbiotic immune” increased the number of CD16+ CD107a+ lymphocytes (activated NK cells) upon antibody stimulation 111.6 ± 34.0% (Figure 4). These results demonstrate that “Postbiotic immune” promotes specifically the proliferation and activation of NK cells.
Figure 4: Bar graphs showing the increase in the number of CD16 + lymphocytes (NK cells) and CD16+ CD107a+ lymphocytes (activated NK cells) before (Day 0) and at the end of the treatment (Day 28) after activation with antibodies. The numbers are represented as % of total cells. Data are presented as mean ± Standard Error of the Mean (SEM). Statistical significance is depicted as **p-value<0.01, ***p-value<0.001.
Self-assessment questionnaire
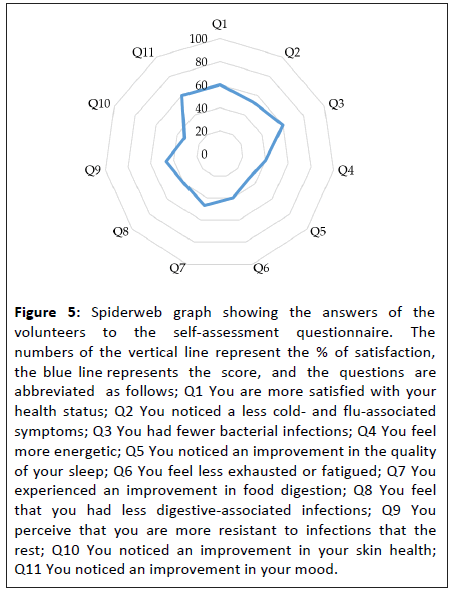
The efficacy of the treatment was subjectively evaluated with a self-assessment questionnaire, including questions about the efficacy of the product (questions 1 to 11) and the personal opinion of the volunteers (questions 12 to 14). All 15 volunteers answered the listed questions with a score ranging from 1 to 5 (1=Strongly Disagree, 2=Disagree, 3=Neutral, 4=Agree, 5=Strongly Agree). For positive impressions, satisfaction is considered when volunteers scored parameters from 4 to 5. Figure 5 shows the results of the answers of the volunteers. Detailed answers of volunteers are shown in Tables S3 and S4.
Figure 5: Spiderweb graph showing the answers of the volunteers to the self-assessment questionnaire. The numbers of the vertical line represent the % of satisfaction, the blue line represents the score, and the questions are abbreviated as follows; Q1 You are more satisfied with your health status; Q2 You noticed a less cold- and flu-associated symptoms; Q3 You had fewer bacterial infections; Q4 You feel more energetic; Q5 You noticed an improvement in the quality of your sleep; Q6 You feel less exhausted or fatigued; Q7 You experienced an improvement in food digestion; Q8 You feel that you had less digestive-associated infections; Q9 You perceive that you are more resistant to infections that the rest; Q10 You noticed an improvement in your skin health; Q11 You noticed an improvement in your mood.
The results obtained in the self-assessment questionnaire regarding the product efficacy (showed in Figure 5 and Tables S3 and S4) are summarized as follows:
60% of the volunteers:
• Were satisfied with their state of health.
• Had fewer bacterial infections.
• Noticed any improvement in their overall mood.
53% of the volunteers:
• Noticed any reduction in cold or flu symptoms.
47% of the volunteers:
• Experienced improvements in digestion.
• Perceived that they were more resistant to infection than the people around them.
40% of the volunteers:
• Felt more energetic.
• Felt less fatigue or exhaustion.
• Felt that they had fewer digestive infections.
33% of the volunteers:
• Noticed improvement in the quality of their sleep.
• Noticed improvement in the health of their skin.
The results obtained in the self-assessment questionnaire regarding the customer opinion (showed in Figure 5 and Tables S3 and S4) are summarized as follows:
The numbers of the vertical line represent the % of satisfaction, the blue line represents the score, and the questions are abbreviated as follows; Q1 you are more satisfied with your health status; Q2 you noticed a less cold- and fluassociated symptoms; Q3 you had fewer bacterial infections; Q4 you feel more energetic; Q5 you noticed an improvement in the quality of your sleep; Q6 you feel less exhausted or fatigued; Q7 you experienced an improvement in food digestion; Q8 you feel that you had less digestive-associated infections; Q9 you perceive that you are more resistant to infections that the rest; Q10 you noticed an improvement in your skin health; Q11 you noticed an improvement in your mood.
• 93% of the volunteers did not experience any negative side effects since they started taking the product.
• 87% of the volunteers would recommend this product to others for improving their immune system.
• 87% of the volunteers would take this product again.
Discussion
Food complements can play a role in supporting and improving overall health when used as part of a balanced diet and a healthy lifestyle. In particular, selenium food complements can improve a variety of body functions, including supporting the immune system, protecting against oxidative damage, and aiding in thyroid hormone metabolism. In this study, we aimed to evaluate the enhancement of the function of the immune system upon complementation with a selenium rich whole cell S. cerevisiae ABB S15, K. marxianus ABB S8 and S. boulardii ABB S3, used as a postbiotic product (postbiotic immune).
Our results showed that oral intake of “Postbiotic immune” for 28 days resulted in an increase of 108.7% in the selenium levels in plasma, demonstrating that the product “Postbiotic immune” is a dietary supplement able to increase plasma selenium levels. These results manifest the possibility of using “Postbiotic immune” as a supplement to correct selenium deficiencies that can lead to health problems such as impaired immune function, cardiovascular effects, reproductive and fertility problems, thyroid dysfunction, neurological symptoms or musculoskeletal abnormalities [33].
It is well documented that selenium dietary complements have a positive impact in the function of the immune system [2,34]. Additionally, numerous studies have manifested the beneficial effects of using S. cerevisiae, K. marxianus or S. boulardii probiotics to induce trained immunity and enhance the immune response against pathogens or external threats [14,16,34-37]. To determine whether our postbiotic solution rich in selenium could perform this function, we compared the activation of different immune cells subpopulations in volunteers before and after oral supplementation with the extract. Purified PBMCs from volunteers were exposed to anti- CD3 and anti-CD28 antibodies to trigger a co-stimulatory signal that mimics αβ-T cell receptor (TCR complex)-mediated activation in vitro. We measured the number of lymphocytes, T helper cells (adaptive immune response) and NK cells (innate immune response) and the proportion of activated cells in each subpopulation.
Importantly we observed that both types of lymphocytes, T helper and NK cells from volunteers that underwent treatment with “Postbiotic immune” have a higher proliferative capacity in response to antibody stimulation compared to those before the treatment. In response to an infection or other specific insults, it is typically beneficial for the body to increase the lymphocyte count, being considered a sign of a robust immune response [35,36]. The immune system is classically divided into innate and adaptive immunity. Adaptive immunity in mammals is characterized by two types of lymphocytes, T lymphocytes (T cells), that identify and destroy infected cells directly, and B lymphocytes (B cells), that produce antibodies that target and neutralize pathogens [35,36]. Together, they coordinate a highly specific immune response by recognizing and eliminating the invading microorganisms or cancer cells [35-36]. Therefore, our finding suggests that “Postbiotic immune” enhances the function of the immune system by increasing the capacity of eliciting a strong and robust immune response.
Among T lymphocytes, there are a number of different subsets that fulfill a variety of functions of the immune response. T helper cells, often referred to as CD4+ CD8- T cells, are a subtype of T lymphocytes that play a central role in the immune system. They serve as orchestrators of the body's immune response, such as assisting other immune cells in their function, are essential for activating B cells to produce antibodies, and activating cytotoxic T cells to destroy infected cells. Interestingly, daily oral intake of “Postbiotic immune” for 28 days increased proliferation of CD4+ lymphocytes by 59.4% upon antibody stimulation (compared to the non-treated control), being this increase of a similar extent to that observed for the total lymphocyte population (55.7%). However, “Postbiotic immune” did not increase the percentage of activated helper cells (CD4+ CD40L+ lymphocytes). These findings indicate that “Postbiotic immune” increases the total number of lymphocytes including T helper cells upon antigenactivation, but it does not increase the activation of T helper cells.
The innate immune system serves as the front line of defense against germs, acts rapidly in an identical and nonspecific way every time the body is exposed to pathogens [37,38]. NK cells are effector lymphocytes of the innate immune system that control several types of tumors and microbial infections and can also mount a form of antigen-specific immunologic memory. Thus, NK cells exert sophisticated biological functions that are attributes of both innate and adaptive immunity [39,40]. Daily oral intake of “Postbiotic immune” for 28 days increased the total number of CD16+ lymphocytes by 127.3% after stimulation (when compared to the non-treated control). This increase is markedly higher than that observed for the total lymphocyte population (55.7%), demonstrating that treatment “Postbiotic immune” specifically increases the population of NK lymphocytes. Most importantly, this increase in the proliferation of CD16+ cells were accompanied by an increase (111.6%) in activated NK cells (CD16+ CD107a+), as described for other selenium supplements [24].
These results suggest that the proportional increase of CD4+ lymphocytes/total lymphocytes and the specific increase of proliferation and activation of CD16+ lymphocytes could prime the immune system to elicit a faster immune response involving these cell types. CD4+ and CD16+ cells participate in a variety of essential processes of the innate and adaptive immune response, such as the production of interferon-gamma, interleukin IL-2, Tumor Necrosis Factor (TNF)-beta, secretion of lysosomes containing perforin and granzymes, induction of apoptosis of targeted cells, intercellular communication, cytotoxic activity against targeted cells, and activation of macrophages and other cell types [21,41-48]. Therefore, “Postbiotic immune” increases proliferation of lymphocytes, but specifically acts through the innate immune response by increasing proliferation of and activating NK lymphocytes. Most importantly, selenium complements have been found to elicit some of these types of immuno stimulatory effects in vivo [21,23,34,49-51], thus supporting our conclusion that the increase in selenium levels in plasma “Postbiotic immune” primes the human immune system in order to elicit a faster and more efficient immune response.
Conclusion
In conclusion, our study shows that daily oral intake of a selenium rich postbiotic, compound of whole cell S. cerevisiae ABB S15, K. marxianus ABB S8 and S. boulardii ABB S3, like “Postbiotic immune” for 28 days is associated with a higher proliferative capacity of total lymphocytes and, specifically, an enhanced proliferation and activation of CD16+ lymphocytes (natural killer cells), therefore priming the immune system to elicit a faster and more efficient innate immune response.
Author Contributions
Conceptualization, Jordi Cuñé Castellana, José Luis Mullor and Carlos de Lecea Flores de Lemus; data curation, Marián Merino and José Luis Mullor; formal analysis, Marián Merino and José Luis Mullor; Investigation, Marián Merino and José Luis Mullor; methodology, Jordi Cuñé Castellana, José Luis Mullor and Carlos de Lecea Flores de Lemus; project administration, José Luis Mullor; supervision, José Luis Mullor; writing – original draft, Justyna Meissner; writing – review & editing, Maria Tintoré Gazulla, Justyna Meissner, Marián Merino and José Luis Mullor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of HOSPITAL UNIVERSITARIO Y POLITÉCNICO LA FE (Date 18/05/2023 and Code number 2023-363-1). The study protocol is in accordance with the Scientific Committee on Consumer Safety (SCCS) guidance. It meets all international standards for research studies involving human subjects, the Good Clinical Practices (ICH-GCP), and World Medical Association.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. The informed consent described reasons for the study, possible adverse effects, associated risks and potential benefits of the treatment, and their limits of liability. The volunteers signed and dated the informed consent document to indicate their authorization to proceed and acknowledge their understanding of the contents before the start of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11): 1273-1280.
[CrossRef] [GoogleScholar] [Indexing]
- Bellinger FP, Raman AV, Reeves MA, Berry MJ (2009) Regulation and function of selenoproteins in human disease. Biochem J 422: 11-22.
[CrossRef] [GoogleScholar] [Indexing]
- Wichman J, Winther KH, Bonnema SJ, Hegedus L (2016) Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: a systematic review and meta-analysis. Thyroid 26(12): 1681-1692.
[CrossRef] [GoogleScholar] [Indexing]
- Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16(7): 705-743
[CrossRef] [GoogleScholar] [Indexing]
- Razaghi A, Poorebrahim M, Sarhan D, Björnstedt M (2021) Selenium stimulates the antitumour immunity: Insights to future research. Eur J Cancer 155: 256-267.
[CrossRef] [GoogleScholar] [Indexing]
- Kuria A, Fang X, Li M, Han H, He J, et al. (2020) Does dietary intake of selenium protect against cancer? A systematic review and meta-analysis of population-based prospective studies. Crit Rev Food Sci Nutr 60(4): 684-694.
[CrossRef] [GoogleScholar] [Indexing]
- Kieliszek M (2019) Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 24(7): 1298.
[CrossRef] [GoogleScholar] [Indexing]
- (2023) Qualified presumption of safety (QPS). EFSA
- Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, et al. (2023) Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 15(1): 2185034.
[CrossRef] [GoogleScholar] [Indexing]
- Vinderola G, Sanders ME, Salminen S (2022) The Concept of Postbiotics. Foods 11(8): 1077.
[CrossRef] [GoogleScholar] [Indexing]
- Pique N, Berlanga M, Miñana-Galbis D (2019) Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int J Mol Sci 20(10): 2534.
[CrossRef] [GoogleScholar] [Indexing]
- Liu Z, Tyo KEJ, Martínez JL, Petranovic D, Nielsen J (2012) Different expression systems for production of recombinant proteins in Saccharomyces cerevisiae. Biotechnol Bioeng 109(5): 1259-1268.
[CrossRef] [GoogleScholar] [Indexing]
- Generoso SV, Viana ML, Santos RG, Arantes RME, Martins FS, et al. (2011) Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed Saccharomyces boulardii. Eur J Nutr 50(4): 261-269.
[CrossRef] [GoogleScholar] [Indexing]
- Stier H, Bischoff SC (2016) Influence of Saccharomyces boulardii CNCM I-745on the gut-associated immune system. Clin Exp Gastroenterol 13(9): 269-279
[CrossRef] [GoogleScholar] [Indexing]
- Rad AH, Maleki LA, Kafil HS, Zavoshti HF, Abbasi A (2020) Postbiotics as novel health-promoting ingredients in functional foods. Health Promot Perspect 10(1): 3-4
[CrossRef] [GoogleScholar] [Indexing]
- Maccaferri S, Klinder A, Brigidi P, Cavina P, Costabile A (2012) Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl Environ Microbiol 78(4): 956-964
[CrossRef] [GoogleScholar] [Indexing]
- Ma C, Hoffmann PR (2021) Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin Cell Dev Biol 115: 54-61
[CrossRef] [GoogleScholar] [Indexing]
- Danese S, Sans M, Fiocchi C (2004) Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev 3(5): 394-400
[CrossRef] [GoogleScholar] [Indexing]
- Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, et al. (2010) Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr 140(6): 1155-1161
[CrossRef] [GoogleScholar] [Indexing]
- Romagnani S (2014) T cell subpopulations. Chem Immunol Allergy 100: 155-164
- Broome CS, McArdle F, Kyle JAM, Andrews F, Lowe NM (2004) An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 80(1): 154-162
[CrossRef] [GoogleScholar] [Indexing]
- Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294(1-2): 15-22
[CrossRef] [GoogleScholar] [Indexing]
- Schumacher LK, Roy M, Wishe HI, Cohen MW, Stotzky G (1996) Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biol Trace Elem Res 52(3): 227-239
[CrossRef] [GoogleScholar] [Indexing]
- Avery JC, Hoffmann PR (2018) Selenium, Selenoproteins, and Immunity. Nutrients 10(9): 1203
[CrossRef] [GoogleScholar] [Indexing]
- Trickett A, Kwan YL (2003) T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods 275(1-2):251-255
[CrossRef] [GoogleScholar] [Indexing]
- Bashour KT, Gondarenko A, Chen H, Shen K, Liu X, et al. (2014) CD28 and CD3 have complementary roles in T-cell traction forces. Proc Natl Acad Sci USA 111(6): 2241-2246
[CrossRef] [GoogleScholar] [Indexing]
- Soltantoye T, Akbari B, Mirzaei HR, Hadjati J (2022) Soluble and Immobilized Anti-CD3/28 Distinctively Expand and Differentiate Primary Human T Cells: An Implication for Adoptive T Cell Therapy. Iran J Allergy Asthma Immunol 21(6): 630-637
[CrossRef] [GoogleScholar] [Indexing]
- Lier RAV, Brouwer M, de Groot ED, Kramer I, Aarden LA, et al. (1991) T cell receptor/CD3 and CD28 use distinct intracellular signaling pathways. Eur J Immunol 21(7):1775-1778
[CrossRef] [GoogleScholar] [Indexing]
- Chen X, Allan DSJ, Krzewski K, Ge B, Kopcow H, et al. (2006) CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci USA 103(27): 10346-10351
[CrossRef] [GoogleScholar] [Indexing]
- Xia F, Qian CR, Xun Z, Hamon Y, Sartre AM, et al. (2018) TCR and CD28 Concomitant Stimulation Elicits a Distinctive Calcium Response in Naive T Cells. Front Immunol 9: 2864
[CrossRef] [GoogleScholar] [Indexing]
- Nandi D, Gross JA, Allison JP (1994) CD28-mediated costimulation is necessary for optimal proliferation of murine NK cells. J Immunol 152(7): 3361-3369
[CrossRef] [GoogleScholar] [Indexing]
- Galea-Lauri J, Darling D, Gan SU, Krivochtchapov L, Kuiper M, et al. (1999) Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol 163(1):62-70
[CrossRef] [GoogleScholar] [Indexing]
- Shreenath AP, Hashmi MF, Dooley J (2018) Selenium Deficiency. StatPearls Publishing
- Meriggi N, Paola MD, Vitali F, Rivero D, Cappa F, et al. (2019) Saccharomyces cerevisiae Induces Immune Enhancing and Shapes Gut Microbiota in Social Wasps. Front Microbiol 10:2320
- Chou WK, Park J, Carey JB, McIntyre DR, Berghman LR (2017) Immunomodulatory Effects of Saccharomyces cerevisiae Fermentation Product Supplementation on Immune Gene Expression and Lymphocyte Distribution in Immune Organs in Broilers. Front Vet Sci 4: 37
[CrossRef] [GoogleScholar] [Indexing]
- Ortuno J, Cuesta A, Rodríguez A, Esteban MA, Meseguer J (2002) Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Vet Immunol Immunopathol 85(1-2): 41-50
[CrossRef] [GoogleScholar] [Indexing]
- Wang W, Li Z, Lv Z, Zhang B, Lv H, et al. (2017) Effects of Kluyveromyces marxianus supplementation on immune responses, intestinal structure and microbiota in broiler chickens. PLoS One 12(7): e0180884
[CrossRef] [GoogleScholar] [Indexing]
- Fabbri M, Smart C, Pardi R (2003) T lymphocytes. Int J Biochem Cell Biol 35(7): 1004-1008
[CrossRef] [GoogleScholar] [Indexing]
- Heinzel S, Marchingo JM, Horton MB, Hodgkin PD (2018) The regulation of lymphocyte activation and proliferation. Curr Opin Immunol 51: 32-38
[CrossRef] [GoogleScholar] [Indexing]
- Luckheeram RV, Zhou R, Verma AD, Xia B (2012) CD4âÂúT cells: differentiation and functions. Clin Dev Immunol 2012:925135
[CrossRef] [GoogleScholar] [Indexing]
- Taguchi T, Mukai K (2019) Innate immunity signalling and membrane trafficking. Curr Opin Cell Biol 59: 1-7
[CrossRef] [GoogleScholar] [Indexing]
- Arneth B (2021) Trained innate immunity. Immunol Res 69(1): 1-7
[CrossRef] [GoogleScholar] [Indexing]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S (2008) Functions of natural killer cells. Nat Immunol 9(5): 503-510
[CrossRef] [GoogleScholar] [Indexing]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, et al. (2011) Innate or adaptive immunity? The example of natural killer cells. Science 331(6013): 44-49
[CrossRef] [GoogleScholar] [Indexing]
- Romagnani S (2000) T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol 85(1): 9-18
[CrossRef] [GoogleScholar] [Indexing]
- Krzewski K, Gil-Krzewska A, Nguyen V, Peruzzi G, Coligan JE (2013) LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood 121(23):4672-4683
[CrossRef] [GoogleScholar] [Indexing]
- Henney CS, Kuribayashi K, Kern DE, Gillis S (1981) Interleukin-2 augments natural killer cell activity. Nature 291(5813): 335-338
[CrossRef] [GoogleScholar] [Indexing]
- Trinchieri G, Perussia B (1985) Immune interferon: a pleiotropic lymphokine with multiple effects. Immunol Today 6(4): 131-136
[CrossRef] [GoogleScholar] [Indexing]
- Roy M, Kiremidjian-Schumacher L, Wishe HI, Cohen MW, Stotzky G (1994) Supplementation with selenium and human immune cell functions: I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol Trace Elem Res 41(1-2): 103-114
[CrossRef] [GoogleScholar] [Indexing]
- Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G (1994) Supplementation with selenium and human immune cell functions: II. Effect on cytotoxic lymphocytes and natural killer cells. Biol Trace Elem Res 41(1-2): 115-127
[CrossRef] [GoogleScholar] [Indexing]
- McKenzie RC, Rafferty TS, Beckett GJ (1998) Selenium: an essential element for immune function. Immunol Today 19(8): 342-345
[CrossRef] [GoogleScholar] [Indexing]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences